Molecular modelling and competition binding study of Br-noscapine and colchicine provide insight into noscapinoid-tubulin binding site
- PMID: 21530342
- PMCID: PMC3091960
- DOI: 10.1016/j.jmgm.2011.03.004
Molecular modelling and competition binding study of Br-noscapine and colchicine provide insight into noscapinoid-tubulin binding site
Erratum in
- J Mol Graph Model. 2011 Sep;30:198
Abstract
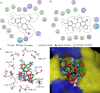
We have previously discovered the tubulin-binding anti-cancer properties of noscapine and its derivatives (noscapinoids). Here, we present three lines of evidence that noscapinoids bind at or near the well studied colchicine binding site of tubulin: (1) in silico molecular docking studies of Br-noscapine and noscapine yield highest docking score with the well characterised colchicine-binding site from the co-crystal structure; (2) the molecular mechanics-generalized Born/surface area (MM-GB/SA) scoring results ΔΔG(bind-cald) for both noscapine and Br-noscapine (3.915 and 3.025 kcal/mol) are in reasonably good agreement with our experimentally determined binding affinity (ΔΔG(bind-Expt) of 3.570 and 2.988 kcal/mol, derived from K(d) values); and (3) Br-noscapine competes with colchicine binding to tubulin. The simplest interpretation of these collective data is that Br-noscapine binds tubulin at a site overlapping with, or very close to colchicine-binding site of tubulin. Although we cannot rule out a formal possibility that Br-noscapine might bind to a site distinct and distant from the colchicine-binding site that might negatively influence the colchicine binding to tubulin.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures




References
-
- Rowinski EK. The development and clinical utility of the taxane class of animicrotubule chemotherapy agents. Annu. Rev. Med. 1997;48:353–374. - PubMed
-
- Jordan MA, Wilson L. The use and action of drugs in analyzing mitosis. Methods Cell Biol. 1999;61:267–295. - PubMed
-
- Checchi PM, Nettles JH, Zhou J, Snyder JP, Joshi HC. Microtubule-interacting drugs for cancer treatment. Trends in Pharmacol. Sci. 2003;24:361–365. - PubMed
-
- Joshi HC, Salil A, Bughani U, Naik PK. Medicinal Plant Biotechnology. CABI South Asia Edition; UK: 2010. Noscapinoids: a new class of anticancer drugs demand biotechnological intervention; pp. 303–320.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

