Functions of autophagy in hepatic and pancreatic physiology and disease
- PMID: 21530520
- PMCID: PMC3690365
- DOI: 10.1053/j.gastro.2011.04.038
Functions of autophagy in hepatic and pancreatic physiology and disease
Abstract
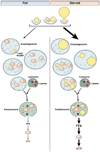
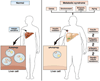
Autophagy is a lysosomal pathway that degrades and recycles intracellular organelles and proteins to maintain energy homeostasis during times of nutrient deprivation and to remove damaged cell components. Recent studies have identified new functions for autophagy under basal and stressed conditions. In the liver and pancreas, autophagy performs the standard functions of degrading mitochondria and aggregated proteins and regulating cell death. In addition, autophagy functions in these organs to regulate lipid accumulation in hepatic steatosis, trypsinogen activation in pancreatitis, and hepatitis virus replication. This review discusses the effects of autophagy on hepatic and pancreatic physiology and the contribution of this degradative process to diseases of these organs. The discovery of novel functions for this lysosomal pathway has increased our understanding of the pathophysiology of diseases in the liver and pancreas and suggested new possibilities for their treatment.
Copyright © 2011 AGA Institute. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflicts of interest
The author discloses no conflicts.
Figures



References
-
- Finn PF, Dice JF. Proteolytic and lipolytic responses to starvation. Nutrition. 2006;22:830–844. - PubMed
-
- Iwata J, Ezaki J, Komatsu M, et al. Excess peroxisomes are degraded by autophagic machinery in mammals. J Biol Chem. 2006;281:4035–4041. - PubMed
-
- Cuervo AM, Knecht E, Terlecky SR, et al. Activation of a selective pathway of lysosomal proteolysis in rat liver by prolonged starvation. Am J Physiol. 1995;269:C1200–C1208. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

