Regulation of nucleocytoplasmic trafficking of viral proteins: an integral role in pathogenesis?
- PMID: 21530593
- PMCID: PMC7114211
- DOI: 10.1016/j.bbamcr.2011.03.019
Regulation of nucleocytoplasmic trafficking of viral proteins: an integral role in pathogenesis?
Abstract
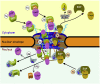
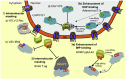
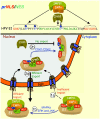
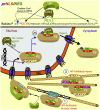
Signal-dependent targeting of proteins into and out of the nucleus is mediated by members of the importin (IMP) family of transport receptors, which recognise targeting signals within a cargo protein and mediate passage through the nuclear envelope-embedded nuclear pore complexes. Regulation of this process is paramount to processes such as cell division and differentiation, but is also critically important for viral replication and pathogenesis; phosphorylation appears to play a major role in regulating viral protein nucleocytoplasmic trafficking, along with other posttranslational modifications. This review focuses on viral proteins that utilise the host cell IMP machinery in order to traffic into/out of the nucleus, and in particular those where trafficking is critical to viral replication and/or pathogenesis, such as simian virus SV40 large tumour antigen (T-ag), human papilloma virus E1 protein, human cytomegalovirus processivity factor ppUL44, and various gene products from RNA viruses such as Rabies. Understanding of the mechanisms regulating viral protein nucleocytoplasmic trafficking is paramount to the future development of urgently needed specific and effective anti-viral therapeutics. This article was originally intended for the special issue "Regulation of Signaling and Cellular Fate through Modulation of Nuclear Protein Import". The Publisher apologizes for any inconvenience caused.
Copyright © 2011 Elsevier B.V. All rights reserved.
Figures


References
-
- Mosammaparast N., Pemberton L.F. Karyopherins: from nuclear-transport mediators to nuclear-function regulators. Trends Cell Biol. 2004;14:547–556. - PubMed
-
- Suntharalingam M., Wente S.R. Peering through the pore: nuclear pore complex structure, assembly, and function. Dev. Cell. 2003;4:775–789. - PubMed
-
- Gorlich D., Kutay U. Transport between the cell nucleus and the cytoplasm. Annu. Rev. Cell Dev. Biol. 1999;15:607–660. - PubMed
-
- Jans D.A., Xiao C.Y., Lam M.H. Nuclear targeting signal recognition: a key control point in nuclear transport? Bioessays. 2000;22:532–544. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

