Control of synapse development and plasticity by Rho GTPase regulatory proteins
- PMID: 21530608
- PMCID: PMC3129138
- DOI: 10.1016/j.pneurobio.2011.04.011
Control of synapse development and plasticity by Rho GTPase regulatory proteins
Abstract
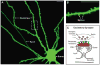
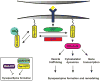
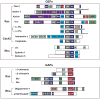
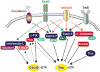
Synapses are specialized cell-cell contacts that mediate communication between neurons. Most excitatory synapses in the brain are housed on dendritic spines, small actin-rich protrusions extending from dendrites. During development and in response to environmental stimuli, spines undergo marked changes in shape and number thought to underlie processes like learning and memory. Improper spine development, in contrast, likely impedes information processing in the brain, since spine abnormalities are associated with numerous brain disorders. Elucidating the mechanisms that regulate the formation and plasticity of spines and their resident synapses is therefore crucial to our understanding of cognition and disease. Rho-family GTPases, key regulators of the actin cytoskeleton, play essential roles in orchestrating the development and remodeling of spines and synapses. Precise spatio-temporal regulation of Rho GTPase activity is critical for their function, since aberrant Rho GTPase signaling can cause spine and synapse defects as well as cognitive impairments. Rho GTPases are activated by guanine nucleotide exchange factors (GEFs) and inhibited by GTPase-activating proteins (GAPs). We propose that Rho-family GEFs and GAPs provide the spatiotemporal regulation and signaling specificity necessary for proper Rho GTPase function based on the following features they possess: (i) existence of multiple GEFs and GAPs per Rho GTPase, (ii) developmentally regulated expression, (iii) discrete localization, (iv) ability to bind to and organize specific signaling networks, and (v) tightly regulated activity, perhaps involving GEF/GAP interactions. Recent studies describe several Rho-family GEFs and GAPs that uniquely contribute to spinogenesis and synaptogenesis. Here, we highlight several of these proteins and discuss how they occupy distinct biochemical niches critical for synaptic development.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures
References
-
- Ahmed S, Lee J, Kozma R, Best A, Monfries C, Lim L. A novel functional target for tumor-promoting phorbol esters and lysophosphatidic acid. The p21rac-GTPase activating protein n-chimaerin. J Biol Chem. 1993;268:10709–10712. - PubMed
-
- Alexander M, Chan KK, Byrne AB, Selman G, Lee T, Ono J, Wong E, Puckrin R, Dixon SJ, Roy PJ. An UNC-40 pathway directs postsynaptic membrane extension in Caenorhabditis elegans. Development. 2009;136:911–922. - PubMed
-
- Allen KM, Gleeson JG, Bagrodia S, Partington MW, MacMillan JC, Cerione RA, Mulley JC, Walsh CA. PAK3 mutation in nonsyndromic X-linked mental retardation. Nat Genet. 1998;20:25–30. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

