The spiral ganglion: connecting the peripheral and central auditory systems
- PMID: 21530629
- PMCID: PMC3152679
- DOI: 10.1016/j.heares.2011.04.003
The spiral ganglion: connecting the peripheral and central auditory systems
Abstract
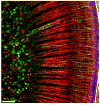
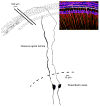
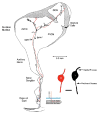
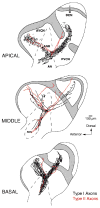
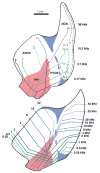
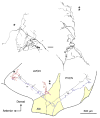
In mammals, the initial bridge between the physical world of sound and perception of that sound is established by neurons of the spiral ganglion. The cell bodies of these neurons give rise to peripheral processes that contact acoustic receptors in the organ of Corti, and the central processes collect together to form the auditory nerve that projects into the brain. In order to better understand hearing at this initial stage, we need to know the following about spiral ganglion neurons: (1) their cell biology including cytoplasmic, cytoskeletal, and membrane properties, (2) their peripheral and central connections including synaptic structure; (3) the nature of their neural signaling; and (4) their capacity for plasticity and rehabilitation. In this report, we will update the progress on these topics and indicate important issues still awaiting resolution.
Copyright © 2011 Elsevier B.V. All rights reserved.
Figures







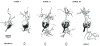
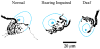


References
-
- Adamo NJ, Daigneault EA. Ultrastructural features of neurons and nerve fibres in the spiral ganglia of cats. J Neurocytol. 1973;2:91–103. - PubMed
-
- Adams JC. Neuronal morphology in the human cochlear nucleus. Arch Otolaryngol Head Neck Surg. 1986;112:1253–1261. - PubMed
-
- Alving BM, Cowan WM. Some quantitative observations on the cochlear division of the eighth nerve in the squirrel monkey (Saimiri sciureus) Brain Res. 1971;25:229–239. - PubMed
-
- Araki S, Kawano A, Seldon L, Shepherd RK, Funasaka S, Clark GM. Effects of chronic electrical stimulation on spiral ganglion neuron survival and size in deafened kittens. Laryngoscope. 1998;108:687–695. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

