Ubiquitin-proteasome pathway and cellular responses to oxidative stress
- PMID: 21530648
- PMCID: PMC3109097
- DOI: 10.1016/j.freeradbiomed.2011.03.031
Ubiquitin-proteasome pathway and cellular responses to oxidative stress
Abstract
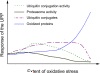
The ubiquitin-proteasome pathway (UPP) is the primary cytosolic proteolytic machinery for the selective degradation of various forms of damaged proteins. Thus, the UPP is an important protein quality control mechanism. In the canonical UPP, both ubiquitin and the 26S proteasome are involved. Substrate proteins of the canonical UPP are first tagged by multiple ubiquitin molecules and then degraded by the 26S proteasome. However, in noncanonical UPP, proteins can be degraded by the 26S or the 20S proteasome without being ubiquitinated. It is clear that a proteasome is responsible for selective degradation of oxidized proteins, but the extent to which ubiquitination is involved in this process remains a subject of debate. Whereas many publications suggest that the 20S proteasome degrades oxidized proteins independent of ubiquitin, there is also solid evidence indicating that ubiquitin and ubiquitination are involved in degradation of some forms of oxidized proteins. A fully functional UPP is required for cells to cope with oxidative stress and the activity of the UPP is also modulated by cellular redox status. Mild or transient oxidative stress up-regulates the ubiquitination system and proteasome activity in cells and tissues and transiently enhances intracellular proteolysis. Severe or sustained oxidative stress impairs the function of the UPP and decreases intracellular proteolysis. Both the ubiquitin-conjugating enzymes and the proteasome can be inactivated by sustained oxidative stress, especially the 26S proteasome. Differential susceptibilities of the ubiquitin-conjugating enzymes and the 26S proteasome to oxidative damage lead to an accumulation of ubiquitin conjugates in cells in response to mild oxidative stress. Thus, increased levels of ubiquitin conjugates in cells seem to be an indicator of mild oxidative stress.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
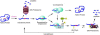
References
-
- Goldberg AL. Protein degradation and protection against misfolded or damaged proteins. Nature. 2003;426:895–899. - PubMed
-
- Ciechanover A. Intracellular protein degradation from a vague idea through the lysosome and the ubiquitin-proteasome system and on to human diseases and drug targeting: Nobel Lecture, December 8, 2004. Ann N Y Acad Sci. 2007;1116:1–28. - PubMed
-
- Glickman MH, Ciechanover A. The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev. 2002;82:373–428. - PubMed
-
- Ciechanover A. The ubiquitin proteolytic system and pathogenesis of human diseases: a novel platform for mechanism-based drug targeting. Biochem Soc Trans. 2003;31:474–481. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

