Structural and functional studies indicating altered redox properties of hemoglobin E: implications for production of bioactive nitric oxide
- PMID: 21531715
- PMCID: PMC3123109
- DOI: 10.1074/jbc.M110.183186
Structural and functional studies indicating altered redox properties of hemoglobin E: implications for production of bioactive nitric oxide
Abstract
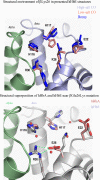
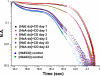
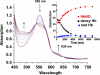
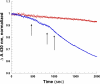
Hemoglobin (Hb) E (β-Glu26Lys) remains an enigma in terms of its contributions to red blood cell (RBC) pathophysiological mechanisms; for example, EE individuals exhibit a mild chronic anemia, and HbE/β-thalassemia individuals show a range of clinical manifestations, including high morbidity and death, often resulting from cardiac dysfunction. The purpose of this study was to determine and evaluate structural and functional consequences of the HbE mutation that might account for the pathophysiology. Functional studies indicate minimal allosteric consequence to both oxygen and carbon monoxide binding properties of the ferrous derivatives of HbE. In contrast, redox-sensitive reactions are clearly impacted as seen in the following: 1) the ∼2.5 times decrease in the rate at which HbE catalyzes nitrite reduction to nitric oxide (NO) relative to HbA, and 2) the accelerated rate of reduction of aquometHbE by L-cysteine (L-Cys). Sol-gel encapsulation studies imply a shift toward a higher redox potential for both the T and R HbE structures that can explain the origin of the reduced nitrite reductase activity of deoxyHbE and the accelerated rate of reduction of aquometHbE by cysteine. Deoxy- and CO HbE crystal structures (derived from crystals grown at or near physiological pH) show loss of hydrogen bonds in the microenvironment of βLys-26 and no significant tertiary conformational perturbations at the allosteric transition sites in the R and T states. Together, these data suggest a model in which the HbE mutation, as a consequence of a relative change in redox properties, decreases the overall rate of Hb-mediated production of bioactive NO.
Figures

References
-
- Giardine B., van Baal S., Kaimakis P., Riemer C., Miller W., Samara M., Kollia P., Anagnou N. P., Chui D. H., Wajcman H., Hardison R. C., Patrinos G. P. (2007) Hum. Mutat. 28, 206 - PubMed
-
- Forget B. H., Higgs D. R. (2009) in Disorders of Hemoglobin: Genetics, Pathophysiology, and Clinical Management (Steinberg M. H., Forget B. G., Higgs D. R., Weatherall D. J. eds) 2nd Ed., pp. 25–26, Cambridge University Press, New York
-
- Chotivanich K., Udomsangpetch R., Pattanapanyasat K., Chierakul W., Simpson J., Looareesuwan S., White N. (2002) Blood 100, 1172–1176 - PubMed
-
- Williams T. N. (2006) Curr. Opin. Microbiol. 9, 388–394 - PubMed
-
- Vichinsky E. (2007) American Society Hematology Education Program, pp. 79–83 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

