EGFL6 promotes endothelial cell migration and angiogenesis through the activation of extracellular signal-regulated kinase
- PMID: 21531721
- PMCID: PMC3121348
- DOI: 10.1074/jbc.M110.187633
EGFL6 promotes endothelial cell migration and angiogenesis through the activation of extracellular signal-regulated kinase
Abstract
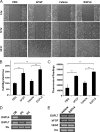
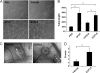
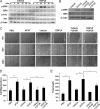
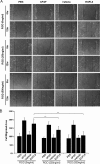
Angiogenesis is required for bone development, growth, and repair. It is influenced by the local bone environment that involves cross-talks between endothelial cells and adjacent bone cells. However, data regarding factors that directly contribute to angiogenesis by bone cells remain poorly understood. Here, we report that EGFL6, a member of the epidermal growth factor (EGF) repeat superfamily proteins, induces angiogenesis by a paracrine mechanism in which EGFL6 is expressed in osteoblastic-like cells but promotes migration and angiogenesis of endothelial cells. Co-immunoprecipitation assays revealed that EGFL6 is secreted in culture medium as a homodimer protein. Using scratch wound healing and transwell assays, we found that conditioned medium containing EGFL6 potentiates SVEC (a simian virus 40-transformed mouse microvascular endothelial cell line) endothelial cell migration. In addition, EGFL6 promotes the endothelial cell tube-like structure formation in Matrigel assays and angiogenesis in a chick embryo chorioallantoic membrane. Furthermore, we show that EGFL6 recombinant protein induces phosphorylation of ERK in SVEC endothelial cells. Inhibition of ERK impaired EGFL6-induced ERK activation and endothelial cell migration. Together, these results demonstrate, for the first time, that osteoblastic-like cells express EGFL6 that is capable of promoting endothelial cell migration and angiogenesis via ERK activation. Thus, the EGLF6 mediates a paracrine mechanism of cross-talk between vascular endothelial cells and osteoblasts and might offer an important new target for the potential treatment of bone diseases, including osteonecrosis, osteoporosis, and fracture healing.
Figures


Similar articles
-
EGFL7 is expressed in bone microenvironment and promotes angiogenesis via ERK, STAT3, and integrin signaling cascades.J Cell Physiol. 2015 Jan;230(1):82-94. doi: 10.1002/jcp.24684. J Cell Physiol. 2015. PMID: 24909139
-
NPNT is Expressed by Osteoblasts and Mediates Angiogenesis via the Activation of Extracellular Signal-regulated Kinase.Sci Rep. 2016 Oct 26;6:36210. doi: 10.1038/srep36210. Sci Rep. 2016. PMID: 27782206 Free PMC article.
-
Osteoblast-derived EGFL6 couples angiogenesis to osteogenesis during bone repair.Theranostics. 2021 Sep 27;11(20):9738-9751. doi: 10.7150/thno.60902. eCollection 2021. Theranostics. 2021. PMID: 34815781 Free PMC article.
-
EGFL6 activates the ERK signaling to improve angiogenesis and osteogenesis of BMSCs in patients with steroid-induced osteonecrosis of the femoral head.Naunyn Schmiedebergs Arch Pharmacol. 2024 Jun;397(6):4287-4298. doi: 10.1007/s00210-023-02880-0. Epub 2023 Dec 12. Naunyn Schmiedebergs Arch Pharmacol. 2024. PMID: 38085290
-
The emerging role of EGFL6 in angiogenesis and tumor progression.Int J Med Sci. 2020 May 25;17(10):1320-1326. doi: 10.7150/ijms.45129. eCollection 2020. Int J Med Sci. 2020. PMID: 32624687 Free PMC article. Review.
Cited by
-
Epidermal growth factor-like domain multiple 6 (EGFL6) promotes the migration and invasion of gastric cancer cells by inducing epithelial-mesenchymal transition.Invest New Drugs. 2021 Apr;39(2):304-316. doi: 10.1007/s10637-020-01004-2. Epub 2020 Sep 19. Invest New Drugs. 2021. PMID: 32949323
-
Single-Cell Transcriptomics Reveals the Complexity of the Tumor Microenvironment of Treatment-Naive Osteosarcoma.Front Oncol. 2021 Jul 21;11:709210. doi: 10.3389/fonc.2021.709210. eCollection 2021. Front Oncol. 2021. PMID: 34367994 Free PMC article.
-
Nephronectin as a Matrix Effector in Cancer.Cancers (Basel). 2021 Feb 25;13(5):959. doi: 10.3390/cancers13050959. Cancers (Basel). 2021. PMID: 33668838 Free PMC article. Review.
-
Identification of a gene expression driven progression pathway in myxoid liposarcoma.Oncotarget. 2014 Aug 15;5(15):5965-77. doi: 10.18632/oncotarget.2023. Oncotarget. 2014. PMID: 25115389 Free PMC article.
-
Silk-Derived Protein Enhances Corneal Epithelial Migration, Adhesion, and Proliferation.Invest Ophthalmol Vis Sci. 2017 Mar 1;58(3):1425-1433. doi: 10.1167/iovs.16-19957. Invest Ophthalmol Vis Sci. 2017. PMID: 28257533 Free PMC article.
References
-
- Brandi M. L., Collin-Osdoby P. (2006) J. Bone Miner. Res. 21, 183–192 - PubMed
-
- Gerber H. P., Ferrara N. (2000) Trends Cardiovasc. Med. 10, 223–228 - PubMed
-
- Carlevaro M. F., Cermelli S., Cancedda R., Descalzi Cancedda F. (2000) J. Cell Sci. 113, 59–69 - PubMed
-
- Hauge E. M., Qvesel D., Eriksen E. F., Mosekilde L., Melsen F. (2001) J. Bone Miner. Res. 16, 1575–1582 - PubMed
-
- Parfitt A. M. (2001) J. Bone Miner. Res. 16, 1583–1585 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

