A20 is induced by Kaposi sarcoma-associated herpesvirus-encoded viral FLICE inhibitory protein (vFLIP) K13 and blocks K13-induced nuclear factor-kappaB in a negative feedback manner
- PMID: 21531730
- PMCID: PMC3122214
- DOI: 10.1074/jbc.M111.224048
A20 is induced by Kaposi sarcoma-associated herpesvirus-encoded viral FLICE inhibitory protein (vFLIP) K13 and blocks K13-induced nuclear factor-kappaB in a negative feedback manner
Abstract
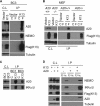
Expression of A20, a negative regulator of the NF-κB pathway, is frequently lost in several subtypes of Hodgkin and non-Hodgkin lymphoma. We report that A20 is expressed in Kaposi sarcoma-associated herpesvirus (KSHV)-infected primary effusion lymphoma cell lines, and its expression correlates closely with the expression of KSHV-encoded viral FLICE inhibitory protein K13. Ectopic expression of K13 induced A20 expression through NF-κB-mediated activation of A20 promoter. In turn, A20 blocked K13-induced NF-κB activity and up-regulation of proinflammatory cytokines CCL20 and IL-8 in a negative feedback fashion. Both the N-terminal deubiquitinating domain and the C-terminal zinc finger domain of A20 were involved in the inhibition of K13-induced NF-κB activity. Overexpression of A20 blocked K13-induced IκBα phosphorylation, NF-κB nuclear translocation, and cellular transformation. Consistent with the above, K13-induced IκBα phosphorylation and NF-κB transcriptional activation were enhanced in A20-deficient cells. Finally, A20 was found to interact physically with K13. Taken collectively, these results demonstrate that K13 is a key determinant of A20 expression in KSHV-infected cells, and A20 is a key negative regulator of K13-induced NF-κB activity. A20 might serve to control the inflammatory response to KSHV infection and protect KSHV-infected cells from apoptosis.
Figures




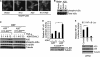
Similar articles
-
A20/TNFAIP3 inhibits NF-κB activation induced by the Kaposi's sarcoma-associated herpesvirus vFLIP oncoprotein.Oncogene. 2013 Mar 7;32(10):1223-32. doi: 10.1038/onc.2012.145. Epub 2012 Apr 23. Oncogene. 2013. PMID: 22525270 Free PMC article.
-
Induction of CCL20 production by Kaposi sarcoma-associated herpesvirus: role of viral FLICE inhibitory protein K13-induced NF-kappaB activation.Blood. 2009 May 28;113(22):5660-8. doi: 10.1182/blood-2008-10-186403. Epub 2009 Mar 26. Blood. 2009. PMID: 19324905 Free PMC article.
-
Kaposi's sarcoma associated herpesvirus encoded viral FLICE inhibitory protein K13 activates NF-κB pathway independent of TRAF6, TAK1 and LUBAC.PLoS One. 2012;7(5):e36601. doi: 10.1371/journal.pone.0036601. Epub 2012 May 8. PLoS One. 2012. PMID: 22590573 Free PMC article.
-
ABINs: A20 binding inhibitors of NF-kappa B and apoptosis signaling.Biochem Pharmacol. 2009 Jul 15;78(2):105-14. doi: 10.1016/j.bcp.2009.02.009. Epub 2009 Feb 27. Biochem Pharmacol. 2009. PMID: 19464428 Review.
-
Cystic Fibrosis from Laboratory to Bedside: The Role of A20 in NF-κB-Mediated Inflammation.Med Princ Pract. 2015;24(4):301-10. doi: 10.1159/000381423. Epub 2015 Apr 25. Med Princ Pract. 2015. PMID: 25925366 Free PMC article. Review.
Cited by
-
Kaposi's sarcoma herpesvirus viral FLICE inhibitory protein modulates A20 deubiquitinase activity.Access Microbiol. 2024 May 9;6(5):000625.v4. doi: 10.1099/acmi.0.000625.v4. eCollection 2024. Access Microbiol. 2024. PMID: 38868372 Free PMC article.
-
A computational profiling of changes in gene expression and transcription factors induced by vFLIP K13 in primary effusion lymphoma.PLoS One. 2012;7(5):e37498. doi: 10.1371/journal.pone.0037498. Epub 2012 May 18. PLoS One. 2012. PMID: 22624040 Free PMC article.
-
Hepatitis C Virus Induces the Ubiquitin-Editing Enzyme A20 via Depletion of the Transcription Factor Upstream Stimulatory Factor 1 To Support Its Replication.mBio. 2019 Jul 23;10(4):e01660-19. doi: 10.1128/mBio.01660-19. mBio. 2019. PMID: 31337730 Free PMC article.
-
Nm23-H1 induces apoptosis in primary effusion lymphoma cells via inhibition of NF-κB signaling through interaction with oncogenic latent protein vFLIP K13 of Kaposi's sarcoma-associated herpes virus.Cell Oncol (Dordr). 2022 Oct;45(5):967-989. doi: 10.1007/s13402-022-00701-9. Epub 2022 Aug 14. Cell Oncol (Dordr). 2022. PMID: 35964258
-
A20/TNFAIP3 inhibits NF-κB activation induced by the Kaposi's sarcoma-associated herpesvirus vFLIP oncoprotein.Oncogene. 2013 Mar 7;32(10):1223-32. doi: 10.1038/onc.2012.145. Epub 2012 Apr 23. Oncogene. 2013. PMID: 22525270 Free PMC article.
References
-
- Aggarwal B. B. (2004) Cancer Cell 6, 203–208 - PubMed
-
- Dixit V. M., Green S., Sarma V., Holzman L. B., Wolf F. W., O'Rourke K., Ward P. A., Prochownik E. V., Marks R. M. (1990) J. Biol. Chem. 265, 2973–2978 - PubMed
-
- Hymowitz S. G., Wertz I. E. (2010) Nat. Rev. Cancer 10, 332–341 - PubMed
-
- Laherty C. D., Hu H. M., Opipari A. W., Wang F., Dixit V. M. (1992) J. Biol. Chem. 267, 24157–24160 - PubMed
-
- Laherty C. D., Perkins N. D., Dixit V. M. (1993) J. Biol. Chem. 268, 5032–5039 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

