Mucociliary interactions and mucus dynamics in ciliated human bronchial epithelial cell cultures
- PMID: 21531774
- PMCID: PMC3154625
- DOI: 10.1152/ajplung.00321.2010
Mucociliary interactions and mucus dynamics in ciliated human bronchial epithelial cell cultures
Abstract
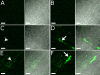
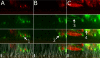
The airway epithelial surface liquid is generally considered to be composed of two layers, a periciliary layer and a continuous thick mucus layer moving in bulk. This view may not be appropriate for all areas of the lung. Our hypothesis, that mucus may form a discontinuous layer with dynamic attachments to the surface, is investigated using a culture system. We used live-cell confocal microscopy to investigate thin mucus layers and fluorescent beads and exogenous MUC5B to visualize mucus dynamics on ciliated human bronchial cultures. A continuous mucus layer was not observed. In sparsely ciliated cultures, mucus attached to ciliated cells; however, in highly ciliated cultures, mucus formed strands several hundred micrometers long. As with increases in ciliation, increases in bead concentration caused the appearance of mucus strands. We confirmed the involvement of mucins in the binding of mucus to cilia by adding labeled purified MUC5B to the cultures. These data suggest that mucins may have an intrinsic ability to form attachments to cilia. The significance of these findings is that aberrant modulation of such an intrinsic property may explain the initiation of highly adherent mucus in cystic fibrosis lung disease.
Figures



References
-
- Armstrong DS, Hook SM, Jamsen KM, Nixon GM, Carzino R, Carlin JB, Robertson CF, Grimwood K. Lower airway inflammation in infants with cystic fibrosis detected by newborn screening. Pediatr Pulmonol 40: 500–510, 2005 - PubMed
-
- Bernacki SH, Nelson AL, Abdullah L, Sheehan JK, Harris A, Davis CW, Randell SH. Mucin gene expression during differentiation of human airway epithelia in vitro. Muc4 and Muc5b are strongly induced. Am J Respir Cell Mol Biol 20: 595–604, 1999 - PubMed
-
- Dillon RH, Fauci LJ, Omoto C, Yang X. Fluid dynamic models of flagellar and ciliary beating. Ann NY Acad Sci 1101: 494–505, 2007 - PubMed
-
- Fulcher ML, Gabriel S, Burns KA, Yankaskas JR, Randell SH. Well-differentiated human airway cultures. Methods Mol Med 107: 183–206, 2005 - PubMed
-
- Gehr P, Green FH, Geiser M, Hof VI, Lee MM, Schürch S. Airway surfactant, a primary defense barrier: mechanical and immunological aspects. J Aerosol Med 9: 163–181, 1996 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

