Modular synthase-encoding gene involved in α-olefin biosynthesis in Synechococcus sp. strain PCC 7002
- PMID: 21531827
- PMCID: PMC3131656
- DOI: 10.1128/AEM.00467-11
Modular synthase-encoding gene involved in α-olefin biosynthesis in Synechococcus sp. strain PCC 7002
Abstract
A gene involved in the production of medium-chain α-olefins was identified in the cyanobacterium Synechococcus sp. strain PCC 7002. The gene encodes a large multidomain protein with homology to type I polyketide synthases, suggesting a route for hydrocarbon biosynthesis from fatty acids via an elongation decarboxylation mechanism.
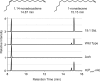
Figures



References
-
- Aparicio J. F., et al. 1996. Organization of the biosynthetic gene cluster for rapamycin in Streptomyces hygroscopicus: analysis of the enzymatic domains in the modular polyketide synthase. Gene 169:9–16 - PubMed
-
- Black P. N., Dirusso C. C., Metzger A. K., Heimert T. L. 1992. Cloning, sequencing, and expression of the fadD gene of Escherichia coli encoding acyl coenzyme-A synthetase. J. Biol. Chem. 267:25513–25520 - PubMed
-
- Chang Z., et al. 2004. Biosynthetic pathway and gene cluster analysis of curacin A, an antitubulin natural product from the tropical marine cyanobacterium Lyngbya majuscula. J. Nat. Prod. 67:1356–1367 - PubMed
-
- Donadio S., Katz L. 1992. Organization of the enzymatic domains in the multifunctional polyketide synthase involved in erythromycin formation in Saccharopolyspora erythraea. Gene 111:51–60 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

