Pannexin channels are not gap junction hemichannels
- PMID: 21532340
- PMCID: PMC3704572
- DOI: 10.4161/chan.5.3.15765
Pannexin channels are not gap junction hemichannels
Abstract
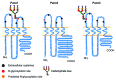
Pannexins, a class of membrane channels, bear significant sequence homology with the invertebrate gap junction proteins, innexins and more distant similarities in their membrane topologies and pharmacological sensitivities with the gap junction proteins, connexins. However, the functional role for the pannexin oligomers, or pannexons, is different from connexin oligomers, the connexons. Many pannexin publications have used the term "hemichannels" to describe pannexin oligomers while others use the term "channels" instead. This has led to confusion within the literature about the function of pannexins that promotes the idea that pannexons serve as gap junction hemichannels and thus have an assembly and functional state as gap junctional intercellular channels. Here we present the case that unlike the connexin gap junction intercellular channels, so far, pannexin oligomers have repeatedly been shown to be channels that are functional in single membranes, but not as intercellular channel in appositional membranes. Hence, they should be referred to as channels and not hemichannels. Thus, we advocate that in the absence of firm evidence that pannexins form gap junctions, the use of the term "hemichannel" be discontinued within the pannexin literature.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
