The value of positive end-expiratory pressure and Fio₂ criteria in the definition of the acute respiratory distress syndrome
- PMID: 21532473
- PMCID: PMC3157575
- DOI: 10.1097/CCM.0b013e31821cb774
The value of positive end-expiratory pressure and Fio₂ criteria in the definition of the acute respiratory distress syndrome
Abstract
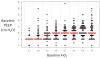
Objectives: The criteria that define acute lung injury and the acute respiratory distress syndrome include PaO₂/Fio₂ but not positive end-expiratory pressure or Fio2. PaO2/Fio2 ratios of some patients increase substantially after mechanical ventilation with positive end-expiratory pressure of 5-10 cm H₂O, and the mortality of these patients may be lower than those whose PaO₂/Fio₂ratios remain <200. Also, PaO₂/Fio₂ may increase when Fio2 is raised from moderate to high levels, suggesting that patients with similar PaO₂/Fio₂ ratios but different Fio₂ levels have different risks of mortality. The primary purpose of this study was to assess the value of adding baseline positive end-expiratory pressure and Fio₂ to PaO₂/Fio₂ for predicting mortality of acute lung injury/acute respiratory distress syndrome patients enrolled in Acute Respiratory Distress Syndrome Network clinical trials. We also assessed effects of two study interventions on clinical outcomes in subsets of patients with mild and severe hypoxemia as defined by PaO₂/Fio₂.
Design: Analysis of baseline physiologic data and outcomes of patients previously enrolled in clinical trials conducted by the National Institutes of Health Acute Respiratory Distress Syndrome Network.
Setting: Intensive care units of 40 hospitals in North America.
Patients: Two thousand three hundred and twelve patients with acute lung injury/acute respiratory distress syndrome.
Interventions: None.
Measurements and main results: Only 1.3% of patients enrolled in Acute Respiratory Distress Syndrome Network trials had baseline positive end-expiratory pressure < 5 cm H₂O, and 50% had baseline positive end-expiratory pressure ≥10 cm H₂O. Baseline PaO₂/Fio₂ predicted mortality, but after controlling for PaO₂/Fio₂, baseline positive end-expiratory pressure did not predict mortality. In contrast, after controlling for baseline PaO₂/Fio₂, baseline Fio₂ did predict mortality. Effects of two study interventions (lower tidal volumes and fluid-conservative hemodynamic management) were similar in mild and severe hypoxemia subsets as defined by PaO₂/Fio₂ ratios.
Conclusion: At Acute Respiratory Distress Syndrome Network hospitals, the addition of baseline positive end-expiratory pressure would not have increased the value of PaO₂/Fio₂ for predicting mortality of acute lung injury/acute respiratory distress syndrome patients. In contrast, the addition of baseline Fio2 to PaO₂/Fio₂ could be used to identify subsets of patients with low or high mortality.
Figures
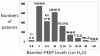



Comment in
-
Towards changing the definition of the acute respiratory distress syndrome: one step forward.Crit Care Med. 2011 Sep;39(9):2177-8. doi: 10.1097/CCM.0b013e318226607a. Crit Care Med. 2011. PMID: 21849824 No abstract available.
References
-
- Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, et al. The American-European Consensus Conference on ARDS. Am J Respir Crit Care Med. 1994;149:818–824. - PubMed
-
- Acute Respiratory Distress Syndrome Network Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. - PubMed
-
- Acute Respiratory Distress Syndorme Network Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004;351(4):327–336. - PubMed
-
- Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, deBoisblanc B, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354(24):2564–2575. - PubMed
-
- Wheeler AP, Bernard GR, Thompson BT, Schoenfeld D, Wiedemann HP, deBoisblanc B, et al. Pulmonary-artery versus central venous catheter to guide treatment of acute lung injury. N Engl J Med. 2006;354(21):2213–2224. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- N01-HR-56169/HR/NHLBI NIH HHS/United States
- N01 HR056170/HR/NHLBI NIH HHS/United States
- N01-HR-56175/HR/NHLBI NIH HHS/United States
- N01-HR-56172/HR/NHLBI NIH HHS/United States
- N01-HR-46059/HR/NHLBI NIH HHS/United States
- N01-HR-46055/HR/NHLBI NIH HHS/United States
- N01-HR-56177/HR/NHLBI NIH HHS/United States
- N01-HR-56166/HR/NHLBI NIH HHS/United States
- N01-HR-56176/HR/NHLBI NIH HHS/United States
- N01-HR-56165/HR/NHLBI NIH HHS/United States
- N01-HR-46060/HR/NHLBI NIH HHS/United States
- N01-HR-46054/HR/NHLBI NIH HHS/United States
- N01-HR-46063/HR/NHLBI NIH HHS/United States
- N01-HR-46064/HR/NHLBI NIH HHS/United States
- N01-HR-46061/HR/NHLBI NIH HHS/United States
- N01-HR-56168/HR/NHLBI NIH HHS/United States
- N01-HR-46057/HR/NHLBI NIH HHS/United States
- N01-HR-56179/HR/NHLBI NIH HHS/United States
- N01-HR-561670/HR/NHLBI NIH HHS/United States
- N01-HR-56171/HR/NHLBI NIH HHS/United States
- N01-HR-46058/HR/NHLBI NIH HHS/United States
- N01-HR-56174/HR/NHLBI NIH HHS/United States
- N01-HR-46056/HR/NHLBI NIH HHS/United States
- N01-HR-56173/HR/NHLBI NIH HHS/United States
- N01-HR-56178/HR/NHLBI NIH HHS/United States
- N01-HR-46062/HR/NHLBI NIH HHS/United States
- N01-HR-56167/HR/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources

