Transcription factor Foxp1 exerts essential cell-intrinsic regulation of the quiescence of naive T cells
- PMID: 21532575
- PMCID: PMC3631322
- DOI: 10.1038/ni.2034
Transcription factor Foxp1 exerts essential cell-intrinsic regulation of the quiescence of naive T cells
Abstract
The molecular mechanisms that underlie T cell quiescence are poorly understood. Here we report that mature naive CD8(+) T cells lacking the transcription factor Foxp1 gained effector phenotype and function and proliferated directly in response to interleukin 7 (IL-7) in vitro. Foxp1 repressed expression of the IL-7 receptor α-chain (IL-7Rα) by antagonizing Foxo1 and negatively regulated signaling by the kinases MEK and Erk. Acute deletion of Foxp1 induced naive T cells to gain an effector phenotype and proliferate in lympho-replete mice. Foxp1-deficient naive CD8(+) T cells proliferated even in lymphopenic mice deficient in major histocompatibility complex class I. Our results demonstrate that Foxp1 exerts essential cell-intrinsic regulation of naive T cell quiescence, providing direct evidence that lymphocyte quiescence is achieved through actively maintained mechanisms that include transcriptional regulation.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

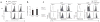

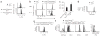

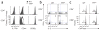

Comment in
-
Fox factors fight over T cell quiescence.Nat Immunol. 2011 Jun;12(6):522-4. doi: 10.1038/ni.2040. Nat Immunol. 2011. PMID: 21587312
References
-
- Yusuf I, Fruman DA. Regulation of quiescence in lymphocytes. Trends Immunol. 2003;24:380–386. - PubMed
-
- Tzachanis D, Lafuente EM, Li L, Boussiotis VA. Intrinsic and extrinsic regulation of T lymphocyte quiescence. Leuk Lymphoma. 2004;45:1959–1967. - PubMed
-
- Ernst B, Lee DS, Chang JM, Sprent J, Surh CD. The peptide ligands mediating positive selection in the thymus control T cell survival and homeostatic proliferation in the periphery. Immunity. 1999;11:173–181. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

