Effectiveness and Patient Acceptance of Halcinonide 0.1% Cream in 216g Jars for Large-area Steroid-responsive Dermatoses
- PMID: 21532875
- PMCID: PMC3084610
Effectiveness and Patient Acceptance of Halcinonide 0.1% Cream in 216g Jars for Large-area Steroid-responsive Dermatoses
Abstract
When treating patients with extensive dermatitis, total body surface area affected must be considered when prescribing topical medication. Halcinonide 0.1% cream, a class 2 topical corticosteroid, is now available in a 216g jar. This large size is convenient and cost effective for patients with large-area dermatoses.
Objectives: The objectives of this study were to determine the efficacy and patient acceptance of halcinonide in 216g jars for the treatment of large-area dermatoses.
Design: This study was an open-label, noncomparator trial evaluating the clinical outcomes and acceptability of halcinonide in 216g jars. Halcinonide was prescribed twice daily for up to 28 days.
Measurement: Severity of dermatoses was based on investigator observations at the baseline visit and again after 28 days. Patient satisfaction was evaluated based on a questionnaire completed at the conclusion of the study.
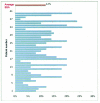
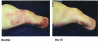
Results: Total enrollment was 40 patients. Dermatoses affected an average of 12 percent body surface area. At baseline, all patients exhibited dermatoses rated as severe or moderate. Nearly half of patients were completely cleared or almost cleared by 28 days, with all patients noting at least some improvement. Most patients agreed that they liked the way the product spread on the skin (94.7%), and more than 80 percent found that it was neither sticky nor greasy. In more than 90 percent of cases, the investigator reported that halcinonide provided a shorter duration of therapy versus triamcinolone one-pound jars.
Conclusion: Halcinonide 0.1% cream in 216g jars is effective and convenient for patients with large-area dermatoses.
Figures




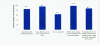
References
-
- Tripodi S. Computer aided design mapping for SCORAD index in atopic dermatitis: ScoradCard software. Pediatr Allergy Immunol. 2005;16(2):182–183. - PubMed
-
- Zeichner JA, Lebwohl MG, Menter A, et al. Optimizing topical therapies for treating psoriasis: a consensus conference. Cutis. 2010;86(Suppl 3):5–31. - PubMed
-
- Halog® [package insert] Princeton, NJ: Ranbaxy Laboratories, Inc; 2009.
LinkOut - more resources
Full Text Sources
