Myelosuppression of thrombocytes and monocytes is associated with a lack of synergy between chemotherapy and anti-VEGF treatment
- PMID: 21532882
- PMCID: PMC3084618
- DOI: 10.1593/neo.101508
Myelosuppression of thrombocytes and monocytes is associated with a lack of synergy between chemotherapy and anti-VEGF treatment
Abstract
Purpose: Chemotherapeutic agents that have shown improved patient outcome when combined with anti-vascular endothelial growth factor (VEGF) therapy were recently identified to induce the mobilization of proangiogenic Tie-2-expressing monocytes (TEMs) and endothelial progenitor cells (EPCs) by platelet release of stromal cell-derived factor 1α (SDF-1α). VEGF blockade was found to counteract cell mobilization. We aimed to determine why agents like gemcitabine do not elicit TEM and EPC recruitment and may therefore lack synergy with anti-VEGF therapy.
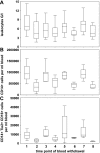
Experimental design: Locally advanced pancreatic cancer patients (n = 20) were monitored during 16 weeks of neoadjuvant therapy. Treatment was based on gemcitabine with or without the addition of bevacizumab. Blood levels of proangiogenic cell populations and angiogenesis factors were determined in 2-week intervals.
Results: The lack of EPC mobilization during gemcitabine therapy was associated with severe thrombocytopenia and reduced SDF-1α blood concentrations. Furthermore, myelosuppression by gemcitabine correlated significantly with loss of TEMs. With respect to angiogenic factors stored and released by platelets, plasma levels of the angiogenesis inhibitor thrombospondin 1 (TSP-1) were selectively decreased and correlated significantly with thrombocytopenia in response to gemcitabine therapy.
Conclusions: A thorough literature screen identified thrombocytopenia as a common feature of chemotherapeutic agents that lack synergy with anti-VEGF treatment. Our results on gemcitabine therapy indicate that myelosuppression (in particular, with respect to thrombocytes and monocytes) interferes with the mobilization of proangiogenic cell types targeted by bevacizumab and may further counteract antiangiogenic therapy by substantially reducing the angiogenesis inhibitor TSP-1.
Figures




References
-
- Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. - PubMed
-
- Kilarski WW, Bikfalvi A. Recent developments in tumor angiogenesis. Curr Pharm Biotechnol. 2007;8:3–9. - PubMed
-
- Verheul HM, Pinedo HM. Vascular endothelial growth factor and its inhibitors. Drugs Today (Barc) 2003;39(suppl C):81–93. - PubMed
-
- Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, Lilenbaum R, Johnson DH. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. - PubMed
-
- Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
