Identification of an exon 4-deletion variant of epidermal growth factor receptor with increased metastasis-promoting capacity
- PMID: 21532887
- PMCID: PMC3084623
- DOI: 10.1593/neo.101744
Identification of an exon 4-deletion variant of epidermal growth factor receptor with increased metastasis-promoting capacity
Abstract
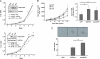
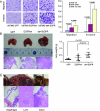
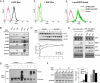
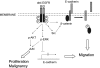
Several types of epidermal growth factor receptor (EGFR) gene alternations have been observed in human tumors. Here we present a novel EGFR variant with aberrant splicing of exon 4 (named as de4 EGFR). Variant-specific polymerase chain reaction showed that de4 EGFR was expressed in some glioma (4/40), prostate cancer (3/11), and ovarian cancer (3/9) tissues but not in tissues adjacent to tumors or normal tissues. de4 EGFR displayed an enhanced transformation and a higher metastasis-promoting capacity in comparison to wild-type EGFR. With minimal EGF-binding activity, de4 EGFR underwent ligand-independent autophosphorylation and self-dimerization. Moreover, in serum-starved condition, de4 EGFR expression in U87 MG cells significantly upregulated the extracellular signal-regulated kinase and AKT phosphorylation and expression of JUN and Src. Importantly, E-cadherin expression was barely detectable in the U87 MG cells expressing de4 EGFR and restored expression of E-cadherin in these cells inhibited their metastatic behaviors. Taken together, we identified a novel EGFR variant with increased metastasis-promoting activity that may become a promising new target for cancer therapy.
Figures



References
-
- Xue C, Wyckoff J, Liang F, Sidani M, Violini S, Tsai K, Zhang Z, Sahai E, Condeelis J, Segall JE. Epidermal growth factor receptor overexpression results in increased tumor cell motility in vivo coordinately with enhanced intravasation and metastasis. Cancer Res. 2006;66:192–197. - PubMed
-
- Pandiella A, Lehvaslaiho H, Magni M, Alitalo K, Meldolesi J. Activation of an EGFR/neu chimeric receptor: early intracellular signals and cell proliferation responses. Oncogene. 1989;4:1299–1305. - PubMed
-
- Liang K, Ang KK, Milas L, Hunter N, Fan Z. The epidermal growth factor receptor mediates radioresistance. Int J Radiat Oncol Biol Phys. 2003;57:246–254. - PubMed
-
- Ho R, Minturn JE, Hishiki T, Zhao H, Wang Q, Cnaan A, Maris J, Evans AE, Brodeur GM. Proliferation of human neuroblastomas mediated by the epidermal growth factor receptor. Cancer Res. 2005;65:9868–9875. - PubMed
-
- Modjtahedi H, Essapen S. Epidermal growth factor receptor inhibitors in cancer treatment: advances, challenges and opportunities. Anticancer Drugs. 2009;20:851–855. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
