Mechanistic aspects of photooxidation of polyhydroxylated molecules on metal oxides
- PMID: 21532934
- PMCID: PMC3083075
- DOI: 10.1021/jp110612s
Mechanistic aspects of photooxidation of polyhydroxylated molecules on metal oxides
Abstract
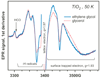
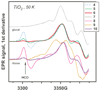
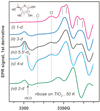
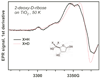
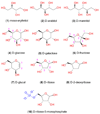
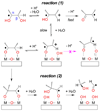
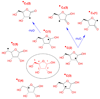
Polyhydroxylated molecules, including natural carbohydrates, are known to undergo photooxidation on wide-gap transition metal oxides irradiated by ultraviolet light. In this study, we examine mechanistic aspects of this photoreaction on aqueous TiO(2), α-FeOOH, and α-Fe(2)O(3) particles using electron paramagnetic resonance (EPR) spectroscopy and site-selective deuteration. We demonstrate that the carbohydrates are oxidized at sites involved in the formation of oxo-bridges between the chemisorbed carbohydrate molecule and metal ions at the oxide surface. This bridging inhibits the loss of water (which is the typical reaction of the analogous free radicals in bulk solvent) promoting instead a rearrangement that leads to elimination of the formyl radical. For natural carbohydrates, the latter reaction mainly involves carbon-1, whereas the main radical products of the oxidation are radical arising from H atom loss centered on carbon-1, -2, and -3 sites. Photoexcited TiO(2) oxidizes all of the carbohydrates and polyols, whereas α-FeOOH oxidizes some of the carbohydrates, and α-Fe(2)O(3) is unreactive. These results serve as a stepping stone for understanding the photochemistry on mineral surfaces of more complex biomolecules such as nucleic acids.
Figures
References
-
- Shkrob IA, Sauer MC. J. Phys. Chem. B. 2004;108:12497.
-
- Shkrob IA, Sauer MC, Gosztola D. J. Phys. Chem. B. 2004;108:12512.
-
- Shkrob IA, Chemerisov SD. J. Phys. Chem. C. 2009;113:17138.
-
- Shkrob IA, Chemerisov SD, Marin TW. Astrobiology. 2010;10:425. - PubMed
-
- Micic OI, Zhang Y, Cromack KR, Trifunac AD, Thurnauer MC. J. Phys. Chem. 1993;97:13284.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
