Drug-loaded, bivalent-bottle-brush polymers by graft-through ROMP
- PMID: 21532937
- PMCID: PMC3083120
- DOI: 10.1021/ma1021506
Drug-loaded, bivalent-bottle-brush polymers by graft-through ROMP
Abstract
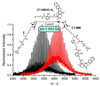
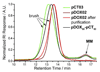
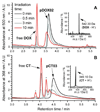
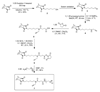
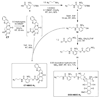
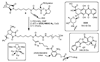
Graft-through ring-opening metathesis polymerization (ROMP) using ruthenium N-heterocyclic carbene catalysts has enabled the synthesis of bottle-brush polymers with unprecedented ease and control. Here we report the first bivalent-brush polymers; these materials were prepared by graft-through ROMP of drug-loaded polyethylene-glycol (PEG) based macromonomers (MMs). Anticancer drugs doxorubicin (DOX) and camptothecin (CT) were attached to a norbornene-alkyne-PEG MM via a photocleavable linker. ROMP of either or both drug-loaded MMs generated brush homo- and co-polymers with low polydispersities and defined molecular weights. Release of free DOX and CT from these materials was initiated by exposure to 365 nm light. All of the CT and DOX polymers were at least 10-fold more toxic to human cancer cells after photoinitiated drug release while a copolymer carrying both CT and DOX displayed 30-fold increased toxicity upon irradiation. Graft-through ROMP of drug-loaded macromonomers provides a general method for the systematic study of structure-function relationships for stimuli-responsive polymers in biological systems.
Figures

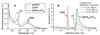


Similar articles
-
Core-clickable PEG-branch-azide bivalent-bottle-brush polymers by ROMP: grafting-through and clicking-to.J Am Chem Soc. 2011 Jan 26;133(3):559-66. doi: 10.1021/ja108441d. Epub 2010 Dec 13. J Am Chem Soc. 2011. PMID: 21142161 Free PMC article.
-
ROMP of Macromonomers Prepared by ROMP: Expanding Access to Complex, Functional Bottlebrush Polymers.J Am Chem Soc. 2025 Jan 29;147(4):3855-3865. doi: 10.1021/jacs.4c17151. Epub 2025 Jan 14. J Am Chem Soc. 2025. PMID: 39808775
-
Polyoxazoline-Based Bottlebrush and Brush-Arm Star Polymers via ROMP: Syntheses and Applications as Organic Radical Contrast Agents.ACS Macro Lett. 2019 Apr 16;8(4):473-478. doi: 10.1021/acsmacrolett.9b00016. Epub 2019 Apr 4. ACS Macro Lett. 2019. PMID: 31289694 Free PMC article.
-
Alkene metathesis - a tool for the synthesis of conjugated polymers.Macromol Rapid Commun. 2012 May 29;33(10):886-910. doi: 10.1002/marc.201200001. Epub 2012 Apr 17. Macromol Rapid Commun. 2012. PMID: 22508469 Review.
-
Functional Precision Polymers via Stereo- and Regioselective Polymerization Using Group 6 Metal Alkylidene and Group 6 and 8 Metal Alkylidene N-Heterocyclic Carbene Complexes.Macromol Rapid Commun. 2019 Jan;40(1):e1800492. doi: 10.1002/marc.201800492. Epub 2018 Aug 17. Macromol Rapid Commun. 2019. PMID: 30118168 Review.
Cited by
-
Shape Control in Engineering of Polymeric Nanoparticles for Therapeutic Delivery.Biomater Sci. 2015 Jul;3(7):894-907. doi: 10.1039/C5BM00006H. Biomater Sci. 2015. PMID: 26146550 Free PMC article.
-
Design and Synthesis of a Cyclic Double-Grafted Polymer Using Active Ester Chemistry and Click Chemistry via A "Grafting onto" Method.Polymers (Basel). 2019 Feb 1;11(2):240. doi: 10.3390/polym11020240. Polymers (Basel). 2019. PMID: 30960224 Free PMC article.
-
Hydrazine-Catalysed Ring-Opening Metathesis Polymerization Of Cyclobutenes.Angew Chem Int Ed Engl. 2024 Dec 20;63(52):e202413093. doi: 10.1002/anie.202413093. Epub 2024 Oct 22. Angew Chem Int Ed Engl. 2024. PMID: 39186258 Free PMC article.
-
Molecular Structure of Foldable Bottlebrush Polymers in Melts.Macromolecules. 2025 Apr 1;58(8):4320-4339. doi: 10.1021/acs.macromol.4c02981. eCollection 2025 Apr 22. Macromolecules. 2025. PMID: 40290573 Free PMC article.
-
Using an RNAi Signature Assay To Guide the Design of Three-Drug-Conjugated Nanoparticles with Validated Mechanisms, In Vivo Efficacy, and Low Toxicity.J Am Chem Soc. 2016 Sep 28;138(38):12494-501. doi: 10.1021/jacs.6b06321. Epub 2016 Sep 14. J Am Chem Soc. 2016. PMID: 27626288 Free PMC article.
References
-
- Hawker CJ, Wooley KL. Science (Washington, DC, U. S.) 2005;309:1200–1205. - PubMed
-
- Hawker CJ, Fokin VV, Finn MG, Sharpless KB. Aust. J. Chem. 2007;60:381–383.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
