Syntheses of meta-[F]Fluorobenzaldehyde and meta-[F]Fluorobenzylbromide from Phenyl(3-Formylphenyl) Iodonium Salt Precursors
- PMID: 21532942
- PMCID: PMC3083861
- DOI: 10.1002/jlcr.1853
Syntheses of meta-[F]Fluorobenzaldehyde and meta-[F]Fluorobenzylbromide from Phenyl(3-Formylphenyl) Iodonium Salt Precursors
Abstract
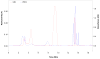
(18)F-labeled fluorobenzaldehydes and fluorobenzylbromides are useful synthons for the preparation of PET radiopharmaceuticals. Whereas ortho- and para-[(18)F]fluorobenzaldehydes can easily be prepared with high yields, the corresponding meta- derivatives are more problematic. In order to improve the yield of meta-[(18)F]fluorobenzaldehyde we used the corresponding diaryliodonium salt precursors, since diaryliodonium salts had already been used as precursors in preparations of (18)F-labeled electron rich, as well as electron deficient, aromatic rings. Diaryliodonium salts with different counter ions [PhIPhCHO]X (X = Cl, Br, OTs, OTf) were synthesized. (18)F radiolabeling was performed using different bases at different temperatures in the presence of a radical scavenger, 2,2,6,6-tetramethylpiperidine-N-oxyl (TEMPO). The best conversion (~80%) to meta-[(18)F] fluorobenzaldehyde was obtained using CsHCO(3) base at a reaction temperature of 110 °C. To study iodonium salt counter ion effects on radiofluorination, each precursor was separately treated with CsF[(18)F]/CsHCO(3) in DMF at 110 °C for 5 min in the presence of TEMPO. Our observed reactivity order was OTs<Cl<OTf<Br. Meta-[(18)F]fluorobenzaldehyde thus obtained was reduced to the corresponding alcohol with aqueous NaBH(4) at room temperature and then converted to meta-[(18)F]fluorobenzylbromide using triphenylphosphine dibromide. Formation of meta-[(18)F]fluorobenzylbromide was confirmed by HPLC and the desired product was purified on a silica Sep-Pak(®) plus cartridge.
Figures
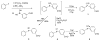
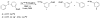

References
-
- Le Bars D. J Fluorine Chem. 2006;127:1488–1493.
-
- Cai L, Lu S, Pike VW. Eur J OrgChem. 2008:2853–2873.
-
- Ametamey SM, Honer M, Schubiger PA. Chem Rev. 2008;108:1501–1516. - PubMed
-
- Papash AI, Alenitsky YG. Phys Part Nucl. 2008;39:597–631.
-
- Atkins HL, Christman DR, Fowler JS, Hauser W, Hoyte RM, Klopper JF, Lin SS, Wolf AP. J Nucl Med. 1972;13:713–719. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

