Functional expression of human adenine nucleotide translocase 4 in Saccharomyces cerevisiae
- PMID: 21532989
- PMCID: PMC3080916
- DOI: 10.1371/journal.pone.0019250
Functional expression of human adenine nucleotide translocase 4 in Saccharomyces cerevisiae
Abstract
The adenine nucleotide translocase (ANT) mediates the exchange of ADP and ATP across the inner mitochondrial membrane. The human genome encodes multiple ANT isoforms that are expressed in a tissue-specific manner. Recently a novel germ cell-specific member of the ANT family, ANT4 (SLC25A31) was identified. Although it is known that targeted depletion of ANT4 in mice resulted in male infertility, the functional biochemical differences between ANT4 and other somatic ANT isoforms remain undetermined. To gain insight into ANT4, we expressed human ANT4 (hANT4) in yeast mitochondria. Unlike the somatic ANT proteins, expression of hANT4 failed to complement an AAC-deficient yeast strain for growth on media requiring mitochondrial respiration. Moreover, overexpression of hANT4 from a multi-copy plasmid interfered with optimal yeast growth. However, mutation of specific amino acids of hANT4 improved yeast mitochondrial expression and supported growth of the AAC-deficient yeast on non-fermentable carbon sources. The mutations affected amino acids predicted to interact with phospholipids, suggesting the importance of lipid interactions for function of this protein. Each mutant hANT4 and the somatic hANTs exhibited similar ADP/ATP exchange kinetics. These data define common and distinct biochemical characteristics of ANT4 in comparison to ANT1, 2 and 3 providing a basis for study of its unique adaptation to germ cells.
Conflict of interest statement
Figures
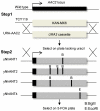
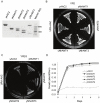


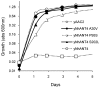

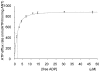

References
-
- Trezeguet V, Pelosi L, Lauquin GJ, Brandolin G. The mitochondrial ADP/ATP carrier:functional and structural studies in the route of elucidating pathophysiological aspects. J Bioenerg Biomembr. 2008;40:435–443. - PubMed
-
- Rodić N, Oka M, Hamazaki T, Murawski M, Jorgensen M, et al. DNA methylation is required for silencing of ant4, an adenine nucleotide translocase selectively expressed in mouse embryonic stem cells and germ cells. Stem Cells. 2005;23:1314–1323. - PubMed
-
- Dolce V, Scarcia P, Iacopetta D, Palmieri F. A fourth ADP/ATP carrier isoform in man:identification, bacterial expression, functional characterization and tissue distribution. FEBS Lett. 2005;579:633–637. - PubMed
-
- Brower JV, Rodic N, Seki T, Jorgensen M, Fliess N, et al. Evolutionarily conserved mammalian adenine nucleotide translocase 4 is essential for spermatogenesis. J Biol Chem. 2007;282:29658–29666. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

