Identification and functional validation of the novel antimalarial resistance locus PF10_0355 in Plasmodium falciparum
- PMID: 21533027
- PMCID: PMC3080868
- DOI: 10.1371/journal.pgen.1001383
Identification and functional validation of the novel antimalarial resistance locus PF10_0355 in Plasmodium falciparum
Abstract
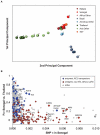
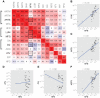
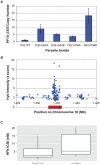
The Plasmodium falciparum parasite's ability to adapt to environmental pressures, such as the human immune system and antimalarial drugs, makes malaria an enduring burden to public health. Understanding the genetic basis of these adaptations is critical to intervening successfully against malaria. To that end, we created a high-density genotyping array that assays over 17,000 single nucleotide polymorphisms (∼ 1 SNP/kb), and applied it to 57 culture-adapted parasites from three continents. We characterized genome-wide genetic diversity within and between populations and identified numerous loci with signals of natural selection, suggesting their role in recent adaptation. In addition, we performed a genome-wide association study (GWAS), searching for loci correlated with resistance to thirteen antimalarials; we detected both known and novel resistance loci, including a new halofantrine resistance locus, PF10_0355. Through functional testing we demonstrated that PF10_0355 overexpression decreases sensitivity to halofantrine, mefloquine, and lumefantrine, but not to structurally unrelated antimalarials, and that increased gene copy number mediates resistance. Our GWAS and follow-on functional validation demonstrate the potential of genome-wide studies to elucidate functionally important loci in the malaria parasite genome.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Volkman SK, Sabeti PC, DeCaprio D, Neafsey DE, Schaffner SF, et al. A genome-wide map of diversity in Plasmodium falciparum. Nat Genet. 2007;39:113–119. - PubMed
-
- Mu J, Awadalla P, Duan J, McGee KM, Keebler J, et al. Genome-wide variation and identification of vaccine targets in the Plasmodium falciparum genome. Nat Genet. 2007:126–130. - PubMed
-
- Carret CK, Horrocks P, Konfortov B, Winzeler E, Qureshi M, et al. Microarray-based comparative genomic analyses of the human malaria parasite Plasmodium falciparum using Affymetrix arrays. Mol Biochem Parasitol. 2005:177–186. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

