Mapping the binding between the tetraspanin molecule (Sjc23) of Schistosoma japonicum and human non-immune IgG
- PMID: 21533061
- PMCID: PMC3080413
- DOI: 10.1371/journal.pone.0019112
Mapping the binding between the tetraspanin molecule (Sjc23) of Schistosoma japonicum and human non-immune IgG
Abstract
Background: Schistosomal parasites can establish parasitization in a human host for decades; evasion of host immunorecognition including surface masking by acquisition of host serum components is one of the strategies explored by the parasites. Parasite molecules anchored on the membrane are the main elements in the interaction. Sjc23, a member of the tetraspanin (TSP) family of Schistosoma japonicum, was previously found to be highly immunogenic and regarded as a vaccine candidate against schistosomiasis. However, studies indicated that immunization with Sjc23 generated rapid antibody responses which were less protective than that with other antigens. The biological function of this membrane-anchored molecule has not been defined after decades of vaccination studies.
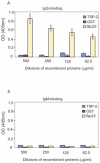
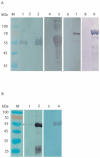
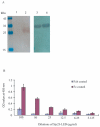
Methodology and principal findings: In this study, we explored affinity pull-down and peptide competition assays to investigate the potential binding between Sjc23 molecule and human non-immune IgG. We determined that Sjc23 could bind human non-immune IgG and the binding was through the interaction of the large extra-cellular domain (LED) of Sjc23 (named Sjc23-LED) with the Fc domain of human IgG. Sjc23 had no affinity to other immunoglobulin types. Affinity precipitation (pull-down assay) in the presence of overlapping peptides further pinpointed to a 9-amino acid motif within Sjc23-LED that mediated the binding to human IgG.
Conclusion and significance: S. japonicum parasites cloak themselves through interaction with human non-immune IgG, and a member of the tetraspanin family, Sjc23, mediated the acquisition of human IgG via the interaction of a motif of 9 amino acids with the Fc domain of the IgG molecule. The consequence of this interaction will likely benefit parasitism of S. japonicum by evasion of host immune recognition or immunoresponses. This is the first report that an epitope of schistosomal ligand and its immunoglobulin receptor are defined, which provides further evidence of immune evasion strategy adopted by S. japonicum.
Conflict of interest statement
Figures



Similar articles
-
[Enhancement of the protective effect of SjC23 DNA vaccine against Schistosoma japonicum infection by immunostimulatory sequence].Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 2005 Feb 28;23(1):1-5. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 2005. PMID: 16042196 Chinese.
-
Protective immunity induced with 23 kDa membrane protein dna vaccine of Schistosoma japonicum Chinese strain in infected C57BL/6 mice.Southeast Asian J Trop Med Public Health. 2003 Dec;34(4):697-701. Southeast Asian J Trop Med Public Health. 2003. PMID: 15115073
-
The protective effect of a Schistosoma japonicum Chinese strain 23 kDa plasmid DNA vaccine in pigs is enhanced with IL-12.Vaccine. 2004 Nov 15;23(1):78-83. doi: 10.1016/j.vaccine.2004.04.031. Vaccine. 2004. PMID: 15519710
-
Identification of novel antigens within the Schistosoma japonicum tetraspanin family based on molecular characterization.Acta Trop. 2011 Mar;117(3):216-24. doi: 10.1016/j.actatropica.2011.01.001. Epub 2011 Jan 13. Acta Trop. 2011. PMID: 21237127
-
Non-immune immunoglobulins shield Schistosoma japonicum from host immunorecognition.Sci Rep. 2015 Aug 24;5:13434. doi: 10.1038/srep13434. Sci Rep. 2015. PMID: 26299686 Free PMC article.
Cited by
-
Evolution of tetraspanin antigens in the zoonotic Asian blood fluke Schistosoma japonicum.Parasit Vectors. 2023 Mar 14;16(1):97. doi: 10.1186/s13071-023-05706-3. Parasit Vectors. 2023. PMID: 36918965 Free PMC article.
-
Functional consequences of allotypic polymorphisms in human immunoglobulin G subclasses.Immunogenetics. 2023 Feb;75(1):1-16. doi: 10.1007/s00251-022-01272-7. Epub 2022 Jul 29. Immunogenetics. 2023. PMID: 35904629 Free PMC article.
-
Comparative genomics and transcriptomics of 4 Paragonimus species provide insights into lung fluke parasitism and pathogenesis.Gigascience. 2020 Jul 1;9(7):giaa073. doi: 10.1093/gigascience/giaa073. Gigascience. 2020. PMID: 32687148 Free PMC article.
-
Cloning, expression and characterisation of a type II cystatin from Schistosoma japonicum, which could regulate macrophage activation.Parasitol Res. 2014 Nov;113(11):3985-92. doi: 10.1007/s00436-014-4064-9. Epub 2014 Aug 6. Parasitol Res. 2014. PMID: 25096535
-
Distinct regions of the large extracellular domain of tetraspanin CD9 are involved in the control of human multinucleated giant cell formation.PLoS One. 2014 Dec 31;9(12):e116289. doi: 10.1371/journal.pone.0116289. eCollection 2014. PLoS One. 2014. PMID: 25551757 Free PMC article.
References
-
- Fenwick A, Webster JP. Schistosomiasis: challenges for control, treatment and drug resistance. Curr Opin Infect Dis. 2006;19:577–582. - PubMed
-
- He YX, Salafsky B, Ramaswamy K. Host—parasite relationships of Schistosoma japonicum in mammalian hosts. Trends Parasitol. 2001;17:320–324. - PubMed
-
- Zhou D, Li Y, Yang X. Schistosomiasis control in China. World Health Forum. 1994;15:387–389. - PubMed
-
- Bergquist R, Al-Sherbiny M, Barakat R, Olds R. Blueprint for schistosomiasis vaccine development. Acta Trop. 2002;82:183–192. - PubMed
-
- Loukas A, Tran M, Pearson MS. Schistosome membrane proteins as vaccines. Int J Parasitol. 2007;37:257–263. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

