Respiratory syncytial virus interferon antagonist NS1 protein suppresses and skews the human T lymphocyte response
- PMID: 21533073
- PMCID: PMC3080852
- DOI: 10.1371/journal.ppat.1001336
Respiratory syncytial virus interferon antagonist NS1 protein suppresses and skews the human T lymphocyte response
Abstract
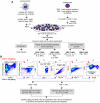
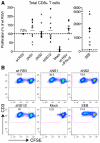
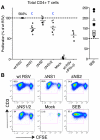
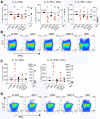
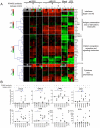
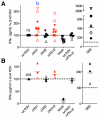
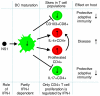
We recently demonstrated that the respiratory syncytial virus (RSV) NS1 protein, an antagonist of host type I interferon (IFN-I) production and signaling, has a suppressive effect on the maturation of human dendritic cells (DC) that was only partly dependent on released IFN-I. Here we investigated whether NS1 affects the ability of DC to activate CD8+ and CD4+ T cells. Human DC were infected with RSV deletion mutants lacking the NS1 and/or NS2 genes and assayed for the ability to activate autologous T cells in vitro, which were analyzed by multi-color flow cytometry. Deletion of the NS1, but not NS2, protein resulted in three major effects: (i) an increased activation and proliferation of CD8+ T cells that express CD103, a tissue homing integrin that directs CD8+ T cells to mucosal epithelial cells of the respiratory tract and triggers cytolytic activity; (ii) an increased activation and proliferation of Th17 cells, which have recently been shown to have anti-viral effects and also indirectly attract neutrophils; and (iii) decreased activation of IL-4-producing CD4+ T cells--which are associated with enhanced RSV disease--and reduced proliferation of total CD4+ T cells. Except for total CD4+ T cell proliferation, none of the T cell effects appeared to be due to increased IFN-I signaling. In the infected DC, deletion of the NS1 and NS2 genes strongly up-regulated the expression of cytokines and other molecules involved in DC maturation. This was partly IFN-I-independent, and thus might account for the T cell effects. Taken together, these data demonstrate that the NS1 protein suppresses proliferation and activation of two of the protective cell populations (CD103+ CD8+ T cells and Th17 cells), and promotes proliferation and activation of Th2 cells that can enhance RSV disease.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Garcia-Sastre A, Egorov A, Matassov D, Brandt S, Levy DE, et al. Influenza A virus lacking the NS1 gene replicates in interferon-deficient systems. Virology. 1998;252:324–330. - PubMed
-
- Tough DF. Type I interferon as a link between innate and adaptive immunity through dendritic cell stimulation. Leuk Lymphoma. 2004;45:257–264. - PubMed
-
- Fujita H, Asahina A, Tada Y, Fujiwara H, Tamaki K. Type I interferons inhibit maturation and activation of mouse Langerhans cells. J Invest Dermatol. 2005;125:126–133. - PubMed
-
- von Hoegen P, Zawatzky R, Schirrmacher V. Modification of tumor cells by a low dose of Newcastle disease virus. III. Potentiation of tumor-specific cytolytic T cell activity via induction of interferon-alpha/beta. Cell Immunol. 1990;126:80–90. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

