NK cell receptor/H2-Dk-dependent host resistance to viral infection is quantitatively modulated by H2q inhibitory signals
- PMID: 21533075
- PMCID: PMC3080855
- DOI: 10.1371/journal.pgen.1001368
NK cell receptor/H2-Dk-dependent host resistance to viral infection is quantitatively modulated by H2q inhibitory signals
Abstract
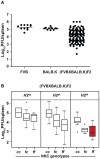
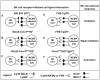
The cytomegalovirus resistance locus Cmv3 has been linked to an epistatic interaction between two loci: a Natural Killer (NK) cell receptor gene and the major histocompatibility complex class I (MHC-I) locus. To demonstrate the interaction between Cmv3 and H2(k), we generated double congenic mice between MA/My and BALB.K mice and an F(2) cross between FVB/N (H-2(q)) and BALB.K (H2(k)) mice, two strains susceptible to mouse cytomegalovirus (MCMV). Only mice expressing H2(k) in conjunction with Cmv3(MA/My) or Cmv3(FVB) were resistant to MCMV infection. Subsequently, an F(3) cross was carried out between transgenic FVB/H2-D(k) and MHC-I deficient mice in which only the progeny expressing Cmv3(FVB) and a single H2-D(k) class-I molecule completely controlled MCMV viral loads. This phenotype was shown to be NK cell-dependent and associated with subsequent NK cell proliferation. Finally, we demonstrated that a number of H2(q) alleles influence the expression level of H2(q) molecules, but not intrinsic functional properties of NK cells; viral loads, however, were quantitatively proportional to the number of H2(q) alleles. Our results support a model in which H-2(q) molecules convey Ly49-dependent inhibitory signals that interfere with the action of H2-D(k) on NK cell activation against MCMV infection. Thus, the integration of activating and inhibitory signals emanating from various MHC-I/NK cell receptor interactions regulates NK cell-mediated control of viral load.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
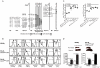



References
-
- Guan H, Moretto M, Bzik DJ, Gigley J, Khan IA. NK cells enhance dendritic cell response against parasite antigens via NKG2D pathway. J Immunol. 2007;179:590–596. - PubMed
-
- Vankayalapati R, Garg A, Porgador A, Griffith DE, Klucar P, et al. Role of NK cell-activating receptors and their ligands in the lysis of mononuclear phagocytes infected with an intracellular bacterium. J Immunol. 2005;175:4611–4617. - PubMed
-
- Gonzalez VD, Landay AL, Sandberg JK. Innate immunity and chronic immune activation in HCV/HIV-1 co-infection. Clin Immunol. 2010;135:12–25. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

