Evolution meets disease: penetrance and functional epistasis of mitochondrial tRNA mutations
- PMID: 21533077
- PMCID: PMC3080857
- DOI: 10.1371/journal.pgen.1001379
Evolution meets disease: penetrance and functional epistasis of mitochondrial tRNA mutations
Abstract
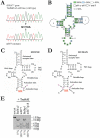
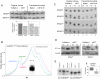
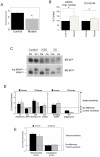
About half of the mitochondrial DNA (mtDNA) mutations causing diseases in humans occur in tRNA genes. Particularly intriguing are those pathogenic tRNA mutations than can reach homoplasmy and yet show very different penetrance among patients. These mutations are scarce and, in addition to their obvious interest for understanding human pathology, they can be excellent experimental examples to model evolution and fixation of mitochondrial tRNA mutations. To date, the only source of this type of mutations is human patients. We report here the generation and characterization of the first mitochondrial tRNA pathological mutation in mouse cells, an m.3739G>A transition in the mitochondrial mt-Ti gene. This mutation recapitulates the molecular hallmarks of a disease-causing mutation described in humans, an m.4290T>C transition affecting also the human mt-Ti gene. We could determine that the pathogenic molecular mechanism, induced by both the mouse and the human mutations, is a high frequency of abnormal folding of the tRNA(Ile) that cannot be charged with isoleucine. We demonstrate that the cells harboring the mouse or human mutant tRNA have exacerbated mitochondrial biogenesis triggered by an increase in mitochondrial ROS production as a compensatory response. We propose that both the nature of the pathogenic mechanism combined with the existence of a compensatory mechanism can explain the penetrance pattern of this mutation. This particular behavior can allow a scenario for the evolution of mitochondrial tRNAs in which the fixation of two alleles that are individually deleterious can proceed in two steps and not require the simultaneous mutation of both.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

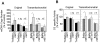




References
-
- Jacobs HT. Disorders of mitochondrial protein synthesis. Hum Mol Genet. 2003;12:R293–301. - PubMed
-
- Chomyn A, Enriquez JA, Micol V, Fernandez-Silva P, Attardi G. The mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episode syndrome-associated human mitochondrial tRNALeu(UUR) mutation causes aminoacylation deficiency and concomitant reduced association of mRNA with ribosomes. J Biol Chem. 2000;275:19198–19209. - PubMed
-
- Enriquez JA, Chomyn A, Attardi G. MtDNA mutation in MERRF syndrome causes defective aminoacylation of tRNA(Lys) and premature translation termination. Nat Genet. 1995;10:47–55. - PubMed
-
- Hao R, Yao YN, Zheng YG, Xu MG, Wang ED. Reduction of mitochondrial tRNALeu(UUR) aminoacylation by some MELAS-associated mutations. FEBS Lett. 2004;578:135–139. - PubMed

