PDP-1 links the TGF-β and IIS pathways to regulate longevity, development, and metabolism
- PMID: 21533078
- PMCID: PMC3080858
- DOI: 10.1371/journal.pgen.1001377
PDP-1 links the TGF-β and IIS pathways to regulate longevity, development, and metabolism
Abstract
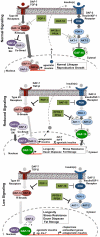
The insulin/IGF-1 signaling (IIS) pathway is a conserved regulator of longevity, development, and metabolism. In Caenorhabditis elegans IIS involves activation of DAF-2 (insulin/IGF-1 receptor tyrosine kinase), AGE-1 (PI 3-kinase), and additional downstream serine/threonine kinases that ultimately phosphorylate and negatively regulate the single FOXO transcription factor homolog DAF-16. Phosphatases help to maintain cellular signaling homeostasis by counterbalancing kinase activity. However, few phosphatases have been identified that negatively regulate the IIS pathway. Here we identify and characterize pdp-1 as a novel negative modulator of the IIS pathway. We show that PDP-1 regulates multiple outputs of IIS such as longevity, fat storage, and dauer diapause. In addition, PDP-1 promotes DAF-16 nuclear localization and transcriptional activity. Interestingly, genetic epistasis analyses place PDP-1 in the DAF-7/TGF-β signaling pathway, at the level of the R-SMAD proteins DAF-14 and DAF-8. Further investigation into how a component of TGF-β signaling affects multiple outputs of IIS/DAF-16, revealed extensive crosstalk between these two well-conserved signaling pathways. We find that PDP-1 modulates the expression of several insulin genes that are likely to feed into the IIS pathway to regulate DAF-16 activity. Importantly, dysregulation of IIS and TGF-β signaling has been implicated in diseases such as Type 2 Diabetes, obesity, and cancer. Our results may provide a new perspective in understanding of the regulation of these pathways under normal conditions and in the context of disease.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Barbieri M, Bonafe M, Franceschi C, Paolisso G. Insulin/IGF-I-signaling pathway: an evolutionarily conserved mechanism of longevity from yeast to humans. Am J Physiol Endocrinol Metab. 2003;285:E1064–1071. - PubMed
-
- Wolkow CA, Munoz MJ, Riddle DL, Ruvkun G. Insulin receptor substrate and p55 orthologous adaptor proteins function in the Caenorhabditis elegans daf-2/insulin-like signaling pathway. J Biol Chem. 2002;277:49591–49597. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

