Modeling the TNFα-induced apoptosis pathway in hepatocytes
- PMID: 21533085
- PMCID: PMC3080376
- DOI: 10.1371/journal.pone.0018646
Modeling the TNFα-induced apoptosis pathway in hepatocytes
Abstract
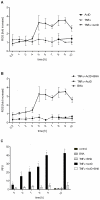
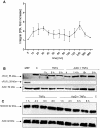
The proinflammatory cytokine TNFα fails to provoke cell death in isolated hepatocytes but has been implicated in hepatocyte apoptosis during liver diseases associated with chronic inflammation. Recently, we showed that TNFα is able to sensitize primary murine hepatocytes cultured on collagen to Fas ligand-induced apoptosis and presented a mathematical model of the sensitizing effect. Here, we analyze how TNFα induces apoptosis in combination with the transcriptional inhibitor actinomycin D (ActD). Accumulation of reactive oxygen species (ROS) in response to TNFR activation turns out to be critical for sustained activation of JNK which then triggers mitochondrial pathway-dependent apoptosis. In addition, the amount of JNK is strongly upregulated in a ROS-dependent way. In contrast to TNFα plus cycloheximide no cFLIP degradation is observed suggesting a different apoptosis pathway in which the Itch-mediated cFLIP degradation and predominantly caspase-8 activation is not involved. Time-resolved data of the respective pro- and antiapoptotic factors are obtained and subjected to mathematical modeling. On the basis of these data we developed a mathematical model which reproduces the complex interplay regulating the phosphorylation status of JNK and generation of ROS. This model was fully integrated with our model of TNFα/Fas ligand sensitizing as well as with a published NF-κB-model. The resulting comprehensive model delivers insight in the dynamical interplay between the TNFα and FasL pathways, NF-κB and ROS and gives an example for successful model integration.
Conflict of interest statement
Figures





References
-
- Canbay A, Friedman S, Gores GJ. Apoptosis: the nexus of liver injury and fibrosis. Hepatology. 2004;39:273–278. - PubMed
-
- Wajant H, Pfizenmaier K, Scheurich P. Tumor necrosis factor signaling. Cell Death Differ. 2003;10:45–65. - PubMed
-
- Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114:181–190. - PubMed
-
- Wullaert A, Heyninck K, Beyaert R. Mechanisms of crosstalk between TNF-induced NF-kappaB and JNK activation in hepatocytes. Biochem Pharmacol. 2006;72:1090–1101. - PubMed
-
- Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

