Modelling the protective efficacy of alternative delivery schedules for intermittent preventive treatment of malaria in infants and children
- PMID: 21533088
- PMCID: PMC3080380
- DOI: 10.1371/journal.pone.0018947
Modelling the protective efficacy of alternative delivery schedules for intermittent preventive treatment of malaria in infants and children
Abstract
Background: Intermittent preventive treatment in infants (IPTi) with sulfadoxine-pyrimethamine (SP) is recommended by WHO where malaria incidence in infancy is high and SP resistance is low. The current delivery strategy is via routine Expanded Program on Immunisation contacts during infancy (EPI-IPTi). However, improvements to this approach may be possible where malaria transmission is seasonal, or where the malaria burden lies mainly outside infancy.
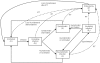
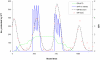
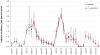
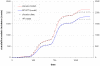
Methods and findings: A mathematical model was developed to estimate the protective efficacy (PE) of IPT against clinical malaria in children aged 2-24 months, using entomological and epidemiological data from an EPI-IPTi trial in Navrongo, Ghana to parameterise the model. The protection achieved by seasonally-targeted IPT in infants (sIPTi), seasonal IPT in children (sIPTc), and by case-management with long-acting artemisinin combination therapies (LA-ACTs) was predicted for Navrongo and for sites with different transmission intensity and seasonality. In Navrongo, the predicted PE of sIPTi was 26% by 24 months of age, compared to 16% with EPI-IPTi. sIPTc given to all children under 2 years would provide PE of 52% by 24 months of age. Seasonally-targeted IPT retained its advantages in a range of transmission patterns. Under certain circumstances, LA-ACTs for case-management may provide similar protection to EPI-IPTi. However, EPI-IPTi or sIPT combined with LA-ACTs would be substantially more protective than either strategy used alone.
Conclusion: Delivery of IPT to infants via the EPI is sub-optimal because individuals are not protected by IPT at the time of highest malaria risk, and because older children are not protected. Alternative delivery strategies to the EPI are needed where transmission varies seasonally or the malaria burden extends beyond infancy. Long-acting ACTs may also make important reductions in malaria incidence. However, delivery systems must be developed to ensure that both forms of chemoprevention reach the individuals who are most exposed to malaria.
Conflict of interest statement
Figures
References
-
- Aponte JJ, Schellenberg D, Egan A, Breckenridge A, Carneiro I, et al. Efficacy and safety of intermittent preventive treatment with sulfadoxine-pyrimethamine for malaria in African infants: a pooled analysis of six randomised, placebo-controlled trials. Lancet. 2009;374:1533–1542. - PubMed
-
- World Health Organisation. Report of the Technical Consultation on Intermittent Preventive Treatment in Infants (IPTi), Technical Expert Group on Preventive Chemotherapy, 23-24 April 2009 - WHO\HQ, Geneva, Switzerland 2009.

