Reversal of diabetic nephropathy by a ketogenic diet
- PMID: 21533091
- PMCID: PMC3080383
- DOI: 10.1371/journal.pone.0018604
Reversal of diabetic nephropathy by a ketogenic diet
Abstract
Intensive insulin therapy and protein restriction delay the development of nephropathy in a variety of conditions, but few interventions are known to reverse nephropathy. Having recently observed that the ketone 3-beta-hydroxybutyric acid (3-OHB) reduces molecular responses to glucose, we hypothesized that a ketogenic diet, which produces prolonged elevation of 3-OHB, may reverse pathological processes caused by diabetes. To address this hypothesis, we assessed if prolonged maintenance on a ketogenic diet would reverse nephropathy produced by diabetes. In mouse models for both Type 1 (Akita) and Type 2 (db/db) diabetes, diabetic nephropathy (as indicated by albuminuria) was allowed to develop, then half the mice were switched to a ketogenic diet. After 8 weeks on the diet, mice were sacrificed to assess gene expression and histology. Diabetic nephropathy, as indicated by albumin/creatinine ratios as well as expression of stress-induced genes, was completely reversed by 2 months maintenance on a ketogenic diet. However, histological evidence of nephropathy was only partly reversed. These studies demonstrate that diabetic nephropathy can be reversed by a relatively simple dietary intervention. Whether reduced glucose metabolism mediates the protective effects of the ketogenic diet remains to be determined.
Conflict of interest statement
Figures


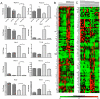
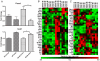

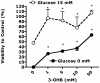
References
-
- Crofford OB. Diabetes control and complications. Annu Rev Med. 1995;46:267–279. - PubMed
-
- Kowluru RA, Abbas SN, Odenbach S. Reversal of hyperglycemia and diabetic nephropathy: effect of reinstitution of good metabolic control on oxidative stress in the kidney of diabetic rats. J Diabetes Complications. 2004;18:282–288. - PubMed
-
- Hamada Y, Fukagawa M. A possible role of thioredoxin interacting protein in the pathogenesis of streptozotocin-induced diabetic nephropathy. Kobe J Med Sci. 2007;53:53–61. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

