Imatinib treatment induces CD5+ B lymphocytes and IgM natural antibodies with anti-leukemic reactivity in patients with chronic myelogenous leukemia
- PMID: 21533122
- PMCID: PMC3078937
- DOI: 10.1371/journal.pone.0018925
Imatinib treatment induces CD5+ B lymphocytes and IgM natural antibodies with anti-leukemic reactivity in patients with chronic myelogenous leukemia
Abstract
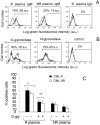
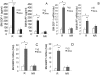
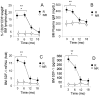
Imatinib mesylate is a first line treatment of Chronic Myelogenous Leukemia and of a rare form of gastrointestinal stromal cancer, where the response to the drug is also linked to the immune system activation with production of antineoplastic cytokines. In this study, forty patients in the chronic phase of disease, treated with imatinib mesylate, were analyzed. Bone marrow aspirates were drawn at diagnosis, after 3, 6, 12, 18 months for haematological, cytofluorimetric, cytogenetic, biomolecular evaluation and cytokine measurement. Responder and non responder patients were defined according to the European LeukemiaNet recommendations. In responder patients (n = 32), the percentage of bone marrow CD20(+)CD5(+)sIgM(+) lymphocytes, and the plasma levels of IgM, were significantly higher, at 3 months and up to 9 months, than in non responders. These IgM reacted with O-linked sugars expressed by leukemic cells and could induce tumor cell apoptosis. In responder patients the stromal-derived factor-1 and the B-lymphocyte-activating factor of the tumor necrosis factor family significantly raised in the bone marrow after imatinib administration, together with the bone morphogenetic proteins-2 and -7. All patients with high number of CD20(+)CD5(+)sIgM(+) cells and high stromal-derived factor-1 and B lymphocyte activating factor levels, underwent complete cytogenetic and/or molecular remission by 12 months. We propose that CD20(+)CD5(+)sIgM(+) lymphocytes producing anti-carbohydrate antibodies with anti-tumor activity, might contribute to the response to imatinib treatment. As in multivariate analysis bone marrow CD20(+)CD5(+)sIgM(+) cells and stromal-derived factor-1 and B-lymphocyte-activating factor levels were significantly related to cytogenetical and molecular changes, they might contribute to the definition of the pharmacological response.
Conflict of interest statement
Figures


References
-
- O'Brien S, Berman E, Borghaei H, Deangelo DJ, Devetten MP, et al. National Comprehensive Cancer Network. NCCN: clinical practice guidelines in oncology. Chronic Myelogenous Leukemia. J Natl Compr Canc. 2009;Netw.7:984–1023. - PubMed
-
- Hochhaus A, O'Brien SG, Guilhot F, Druker BJ, Branford S, et al. Six-year follow-up of patients receiving imatinib for the first-line treatment of chronic myeloid leukaemia. Leukemia. 2009;23:1054–1061. - PubMed
-
- Smyth MJ. Imatinib Mesylate – Uncovering a fast track to adaptive immunity. New Engl J Med. 2006;354:2282–2284. - PubMed
-
- Chan CW, Crafton E, Fan HN, Flook J, Yoshimura K, et al. Interferon-producing killer dendritic cell provide a link between innate and adaptive immunity. Nature Medicine. 2006;12:207–213. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

