Expression of measles virus nucleoprotein induces apoptosis and modulates diverse functional proteins in cultured mammalian cells
- PMID: 21533140
- PMCID: PMC3077409
- DOI: 10.1371/journal.pone.0018765
Expression of measles virus nucleoprotein induces apoptosis and modulates diverse functional proteins in cultured mammalian cells
Abstract
Background: Measles virus nucleoprotein (N) encapsidates the viral RNA, protects it from endonucleases and forms a virus specific template for transcription and replication. It is the most abundant protein during viral infection. Its C-terminal domain is intrinsically disordered imparting it the flexibility to interact with several cellular and viral partners.
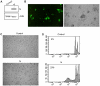
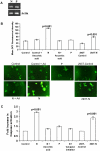
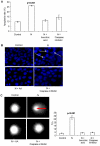
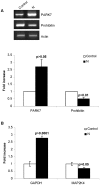
Principal findings: In this study, we demonstrate that expression of N within mammalian cells resulted in morphological transitions, nuclear condensation, DNA fragmentation and activation of Caspase 3 eventuating into apoptosis. The rapid generation of intracellular reactive oxygen species (ROS) was involved in the mechanism of cell death. Addition of ascorbic acid (AA) or inhibitor of caspase-3 in the extracellular medium partially reversed N induced apoptosis. We also studied the protein profile of cells expressing N protein. MS analysis revealed the differential expression of 25 proteins out of which 11 proteins were up regulated while 14 show signs of down regulation upon N expression. 2DE results were validated by real time and semi quantitative RT-PCR analysis.
Conclusion: These results show the pro-apoptotic effects of N indicating its possible development as an apoptogenic tool. Our 2DE results present prima facie evidence that the MV nucleoprotein interacts with or causes differential expression of a wide range of cellular factors. At this stage it is not clear as to what the adaptive response of the host cell is and what reflects a strategic modulation exerted by the virus.
Conflict of interest statement
Figures

References
-
- Griffin DE. Measles virus. In: Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE, editors. Fields Virology. Philadelphia: Lippincott Williams & Wilkins 4th ed; 2001. pp. 1401–1441.
-
- Finch JT, Gibbs AJ. Observations on the structure of the nucleocapsids of some paramyxoviruses. J Gen Virol. 1970;6:141–150. - PubMed
-
- Lamb RA, Kolakofsky D. Paramyxoviridae: The viruses and their replication. In: Fields BN, Knipe DM, Howley PM, editors. Fields Virology. Philadelphia: Lippincott Williams & Wilkins 4th ed; 2001. pp. 1305–1340.
-
- Longhi S, Receveur-Brechot V, Karlin D, Johansson K, Darbon H, et al. The C-terminal domain of the measles virus nucleoprotein is intrinsically disordered and folds upon binding to the C-terminal moiety of the phosphoprotein. J Biol Chem. 2001;278:18638–18648. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

