Exosomes released from M. tuberculosis infected cells can suppress IFN-γ mediated activation of naïve macrophages
- PMID: 21533172
- PMCID: PMC3077381
- DOI: 10.1371/journal.pone.0018564
Exosomes released from M. tuberculosis infected cells can suppress IFN-γ mediated activation of naïve macrophages
Abstract
Background: Macrophages infected with Mycobacterium tuberculosis (M.tb) are known to be refractory to IFN-γ stimulation. Previous studies have shown that M.tb express components such as the 19-kDa lipoprotein and peptidoglycan that can bind to macrophage receptors including the Toll-like receptor 2 resulting in the loss in IFN-γ responsiveness. However, it is unclear whether this effect is limited to infected macrophages. We have previously shown that M.tb-infected macrophages release exosomes which are 30-100 nm membrane bound vesicles of endosomal origin that function in intercellular communication. These exosomes contain mycobacterial components including the 19-kDa lipoprotein and therefore we hypothesized that macrophages exposed to exosomes may show limited response to IFN-γ stimulation.
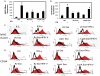
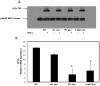
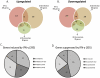
Methodology/principal findings: Exosomes were isolated from resting as well as M.tb-infected RAW264.7 macrophages. Mouse bone marrow-derived macrophages (BMMØ) were treated with exosomes +/- IFN-γ. Cells were harvested and analyzed for suppression of IFN-γ responsive genes by flow cytometry and real time PCR. We found that exosomes derived from M.tb H37Rv-infected but not from uninfected macrophages inhibited IFN-γ induced MHC class II and CD64 expression on BMMØ. This inhibition was only partially dependent on the presence of lipoproteins but completely dependent on TLR2 and MyD88. The exosomes isolated from infected cells did not inhibit STAT1 Tyrosine phosphorylation but down-regulated IFN-γ induced expression of the class II major histocompatibility complex transactivator; a key regulator of class II MHC expression. Microarray studies showed that subsets of genes induced by IFN-γ were inhibited by exosomes from H37Rv-infected cells including genes involved in antigen presentation. Moreover, this set of genes partially overlapped with the IFN-γ-induced genes inhibited by H37Rv infection.
Conclusions: Our study suggests that exosomes, as carriers of M.tb pathogen associated molecular patterns (PAMPs), may provide a mechanism by which M.tb may exert its suppression of a host immune response beyond the infected cell.
Conflict of interest statement
Figures


Similar articles
-
Inhibition of IFN-gamma-induced class II transactivator expression by a 19-kDa lipoprotein from Mycobacterium tuberculosis: a potential mechanism for immune evasion.J Immunol. 2003 Jul 1;171(1):175-84. doi: 10.4049/jimmunol.171.1.175. J Immunol. 2003. PMID: 12816996
-
Prolonged toll-like receptor signaling by Mycobacterium tuberculosis and its 19-kilodalton lipoprotein inhibits gamma interferon-induced regulation of selected genes in macrophages.Infect Immun. 2004 Nov;72(11):6603-14. doi: 10.1128/IAI.72.11.6603-6614.2004. Infect Immun. 2004. PMID: 15501793 Free PMC article.
-
Mycobacterium avium inhibition of IFN-gamma signaling in mouse macrophages: Toll-like receptor 2 stimulation increases expression of dominant-negative STAT1 beta by mRNA stabilization.J Immunol. 2003 Dec 15;171(12):6766-73. doi: 10.4049/jimmunol.171.12.6766. J Immunol. 2003. PMID: 14662881
-
Regulation of antigen presentation by Mycobacterium tuberculosis: a role for Toll-like receptors.Nat Rev Microbiol. 2010 Apr;8(4):296-307. doi: 10.1038/nrmicro2321. Nat Rev Microbiol. 2010. PMID: 20234378 Free PMC article. Review.
-
Antagonistic crosstalk between type I and II interferons and increased host susceptibility to bacterial infections.Virulence. 2010 Sep-Oct;1(5):418-22. doi: 10.4161/viru.1.5.12787. Virulence. 2010. PMID: 21178482 Free PMC article. Review.
Cited by
-
Extracellular vesicles and infectious diseases: new complexity to an old story.J Clin Invest. 2016 Apr 1;126(4):1181-9. doi: 10.1172/JCI81132. Epub 2016 Apr 1. J Clin Invest. 2016. PMID: 27035809 Free PMC article. Review.
-
Mycobacterium tuberculosis TlyA Protein Negatively Regulates T Helper (Th) 1 and Th17 Differentiation and Promotes Tuberculosis Pathogenesis.J Biol Chem. 2015 Jun 5;290(23):14407-17. doi: 10.1074/jbc.M115.653600. Epub 2015 Apr 6. J Biol Chem. 2015. PMID: 25847237 Free PMC article.
-
Message in a vesicle - trans-kingdom intercommunication at the vector-host interface.J Cell Sci. 2019 Mar 18;132(6):jcs224212. doi: 10.1242/jcs.224212. J Cell Sci. 2019. PMID: 30886004 Free PMC article. Review.
-
Extracellular vesicles and chronic inflammation during HIV infection.J Extracell Vesicles. 2019 Nov 6;8(1):1687275. doi: 10.1080/20013078.2019.1687275. eCollection 2019. J Extracell Vesicles. 2019. PMID: 31998449 Free PMC article. Review.
-
Host Long Noncoding RNAs as Key Players in Mycobacteria-Host Interactions.Microorganisms. 2024 Dec 21;12(12):2656. doi: 10.3390/microorganisms12122656. Microorganisms. 2024. PMID: 39770858 Free PMC article. Review.
References
-
- Xie QW, Cho HJ, Calaycay J, Mumford, RA, Swiderek KM, et al. Cloning and characterization of inducible nitric oxide synthase from mouse macrophages. Science. 1992;256:225–228. - PubMed
-
- Siecher SC, Chung GW, Vazquez MA, Lu CY. Augmentation or inhibition of IFN-γ induced MHC class II expression by lipopolysaccharides. The roles of TNF-alpha and nitric oxide and the importance of the sequence of signaling. J Immunol. 1995;155:5826–5834. - PubMed
-
- MacMicking JD, Taylor GA, McKinney JD. Immune control of tuberculosis by 6. IFN-gamma –inducible LRG-47. Science. 2003;302:654–659. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

