Response of estrogen receptor-positive breast cancer tumorspheres to antiestrogen treatments
- PMID: 21533195
- PMCID: PMC3077404
- DOI: 10.1371/journal.pone.0018810
Response of estrogen receptor-positive breast cancer tumorspheres to antiestrogen treatments
Abstract
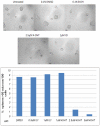
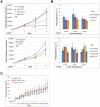
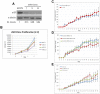
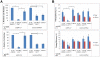
Estrogen signaling plays a critical role in the pathogenesis of breast cancer. Because the majority of breast carcinomas express the estrogen receptor ERα, endocrine therapy that impedes estrogen-ER signaling reduces breast cancer mortality and has become a mainstay of breast cancer treatment. However, patients remain at continued risk of relapse for many years after endocrine treatment. It has been proposed that cancer recurrence may be attributed to cancer stem cells (CSCs)/tumor-initiating cells (TICs). Previous studies in breast cancer have shown that such cells can be enriched and propagated in vitro by culturing the cells in suspension as mammospheres/tumorspheres. Here we established tumorspheres from ERα-positive human breast cancer cell line MCF7 and investigated their response to antiestrogens Tamoxifen and Fulvestrant. The tumorsphere cells express lower levels of ERα and are more tumorigenic in xenograft assays than the parental cells. Both 4-hydroxytamoxifen (4-OHT) and Fulvestrant attenuate tumorsphere cell proliferation, but only 4-OHT at high concentrations interferes with sphere formation. However, treated tumorsphere cells retain the self-renewal capacity. Upon withdrawal of antiestrogens, the treated cells resume tumorsphere formation and their tumorigenic potential remains undamaged. Depletion of ERα shows that ERα is dispensable for tumorsphere formation and xenograft tumor growth in mice. Surprisingly, ERα-depleted tumorspheres display heightened sensitivity to 4-OHT and their sphere-forming capacity is diminished after the drug is removed. These results imply that 4-OHT may inhibit cellular targets besides ERα that are essential for tumorsphere growth, and provide a potential strategy to sensitize tumorspheres to endocrine treatment.
Conflict of interest statement
Figures





References
-
- McDonnell DP, Norris JD. Connections and Regulation of the Human Estrogen Receptor. Science. 2002;296:1642–1964. - PubMed
-
- Anderson WF, Chatterjee N, Ershler WB, Brawley OW. Estrogen receptor breast cancer phenotypes in the Surveillance, Epidemiology, and End Results database. Breast Cancer Res Treat. 2002;76:27–36. - PubMed
-
- Ariazi EA, Ariazi JL, Cordera F, Jordan VC. Estrogen receptors as therapeutic targets in breast cancer. Curr Top Med Chem. 2006;6:181–202. - PubMed
-
- Cleator SJ, Ahamed E, Coombes RC, Palmieri C. A 2009 update on the treatment of patients with hormone receptor-positive breast cancer. Clin Breast Cancer. 2009;9(Suppl):1S6–S17. - PubMed
-
- Sengupta S, Jordan VC. Selective estrogen modulators as an anticancer tool: mechanisms of efficiency and resistance. Adv Exp Med Biol. 2008;630:206–219. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

