Early growth response gene-2 (Egr-2) regulates the development of B and T cells
- PMID: 21533228
- PMCID: PMC3077377
- DOI: 10.1371/journal.pone.0018498
Early growth response gene-2 (Egr-2) regulates the development of B and T cells
Abstract
Background: Understanding of how transcription factors are involved in lymphocyte development still remains a challenge. It has been shown that Egr-2 deficiency results in impaired NKT cell development and defective positive selection of T cells. Here we investigated the development of T, B and NKT cells in Egr-2 transgenic mice and the roles in the regulation of distinct stages of B and T cell development.
Methods and findings: The expression of Egr1, 2 and 3 were analysed at different stages of T and B cell development by RT-PCT and results showed that the expression was strictly regulated at different stages. Forced expression of Egr-2 in CD2(+) lymphocytes resulted in a severe reduction of CD4(+)CD8(+) (DP) cells in thymus and pro-B cells in bone marrow, which was associated with reduced expression of Notch1 in ISP thymocytes and Pax5 in pro-B cells, suggesting that retraction of Egr-2 at the ISP and pro-B cell stages is important for the activation of lineage differentiation programs. In contrast to reduction of DP and pro-B cells, Egr-2 enhanced the maturation of DP cells into single positive (SP) T and NKT cells in thymus, and immature B cells into mature B cells in bone marrow.
Conclusions: Our results demonstrate that Egr-2 expressed in restricted stages of lymphocyte development plays a dynamic, but similar role for the development of T, NKT and B cells.
Conflict of interest statement
Figures


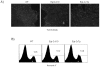


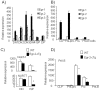
Similar articles
-
The transcription factor early growth response 1 (Egr-1) advances differentiation of pre-B and immature B cells.J Exp Med. 1998 Dec 21;188(12):2215-24. doi: 10.1084/jem.188.12.2215. J Exp Med. 1998. PMID: 9858508 Free PMC article.
-
Two distinct steps during thymocyte maturation from CD4-CD8- to CD4+CD8+ distinguished in the early growth response (Egr)-1 transgenic mice with a recombinase-activating gene-deficient background.J Exp Med. 1997 Sep 15;186(6):877-85. doi: 10.1084/jem.186.6.877. J Exp Med. 1997. PMID: 9294142 Free PMC article.
-
Early growth response (Egr)-1 gene induction in the thymus in response to TCR ligation during early steps in positive selection is not required for CD8 lineage commitment.J Immunol. 2000 Sep 1;165(5):2444-50. doi: 10.4049/jimmunol.165.5.2444. J Immunol. 2000. PMID: 10946269
-
Regulation of intrathymic T-cell development by Lunatic Fringe- Notch1 interactions.Immunol Rev. 2006 Feb;209:76-94. doi: 10.1111/j.0105-2896.2006.00360.x. Immunol Rev. 2006. PMID: 16448535 Review.
-
The role of orphan nuclear receptor in thymocyte differentiation and lymphoid organ development.Immunol Res. 2000;22(2-3):71-82. doi: 10.1385/IR:22:2-3:71. Immunol Res. 2000. PMID: 11339367 Review.
Cited by
-
Involvement of early growth response-2 (Egr-2) in lipopolysaccharide-induced neuroinflammation.J Mol Histol. 2013 Jun;44(3):249-57. doi: 10.1007/s10735-013-9482-y. Epub 2013 Jan 11. J Mol Histol. 2013. PMID: 23307302
-
C/EBPα is required for long-term self-renewal and lineage priming of hematopoietic stem cells and for the maintenance of epigenetic configurations in multipotent progenitors.PLoS Genet. 2014 Jan;10(1):e1004079. doi: 10.1371/journal.pgen.1004079. Epub 2014 Jan 9. PLoS Genet. 2014. PMID: 24415956 Free PMC article.
-
T-cell activation and early gene response in dogs.PLoS One. 2015 Mar 24;10(3):e0121169. doi: 10.1371/journal.pone.0121169. eCollection 2015. PLoS One. 2015. PMID: 25803042 Free PMC article.
-
The ins and outs of type I iNKT cell development.Mol Immunol. 2019 Jan;105:116-130. doi: 10.1016/j.molimm.2018.09.023. Epub 2018 Nov 28. Mol Immunol. 2019. PMID: 30502719 Free PMC article. Review.
-
Three-step transcriptional priming that drives the commitment of multipotent progenitors toward B cells.Genes Dev. 2018 Jan 15;32(2):112-126. doi: 10.1101/gad.309575.117. Epub 2018 Feb 9. Genes Dev. 2018. PMID: 29440259 Free PMC article.
References
-
- Hardy RR, Hayakawa K. B cell development pathways. Annu Rev Immunol. 2001;19:595–621. - PubMed
-
- Carleton M, Haks MC, Smeele SA, Jones A, Belkowski SM, et al. Early growth response transcription factors are required for development of CD4−CD8− thymocytes to the CD4+CD8+ stage. J Immunol. 2002;168:1649–1658. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

