Diet-independent remodeling of cellular membranes precedes seasonally changing body temperature in a hibernator
- PMID: 21533242
- PMCID: PMC3076425
- DOI: 10.1371/journal.pone.0018641
Diet-independent remodeling of cellular membranes precedes seasonally changing body temperature in a hibernator
Abstract
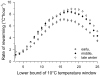
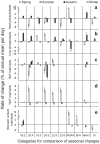
Polyunsaturated fatty acids (PUFA) have a multitude of health effects. Their incorporation into membrane phospholipids (PL) is generally believed to depend directly on dietary influx. PL influence transmembrane protein activity and thus can compensate temperature effects; e.g. PL n-6 PUFA are thought to stabilize heart function at low body temperature (T(b)), whereas long chain (>C18) n-3 PUFA may boost oxidative capacity. We found substantial remodeling of membranes in free-living alpine marmots which was largely independent of direct dietary supply. Organ PL n-6 PUFA and n-6 to n-3 ratios were highest at onset and end of hibernation after rapid increases during a brief transitional period prior to hibernation. In contrast, longer chain PL n-3 PUFA content was low at end of summer but maximal at end of hibernation. After termination of hibernation in spring, these changes in PL composition were rapidly reversed. Our results demonstrate selective trafficking of PUFA within the body, probably governed by a circannual endogenous rhythm, as hibernating marmots were in winter burrows isolated for seven months from food and external cues signaling the approaching spring. High concentrations of PL n-6 PUFA throughout hibernation are in line with their hypothesized function of boosting SERCA 2a activity at low T(b). Furthermore, we found increasing rate of rewarming from torpor during winter indicating increasing oxidative capacity that could be explained by the accumulation of long-chain PL n-3 PUFA. It may serve to minimize the time necessary for rewarming despite the increasing temperature range to be covered, because rewarming is a period of highest metabolic rate and hence production of reactive oxygen species. Considering the importance of PUFA for health our results may have important biomedical implications, as seasonal changes of T(b) and associated remodeling of membranes are not restricted to hibernators but presumably common among endothermic organisms.
Conflict of interest statement
Figures


References
-
- Valencak TG, Arnold W, Tataruch F, Ruf T. High content of polyunsaturated fatty acids in muscle phospholipids of a fast runner, the European brown hare (Lepus europaeus). J Comp Physiol B. 2003;173:695–702. - PubMed
-
- Soriguer FJ, Tinahones FJ, Monzón A, Pareja A, Rojo-Martínez G, et al. Varying incorporation of fatty acids into phospholipids from muscle, adipose and pancreatic exocrine tissues and thymocytes in adult rats fed with diets rich in different fatty acids. Eur J Epidem. 2000;16:585–594. - PubMed
-
- Florant GL. Lipid metabolism in hibernators: the importance of essential fatty acids. Am Zool. 1998;38:331–340.
-
- Florant GL, Amenuddin S, Rintoul DA. Dietary effects on lipid composition and metabolism. In: Romos D, Himms-Hagen J, Suzuki M, editors. Diet and Obesity. Tokyo, Japan: M. Karger; 1991. pp. 45–57.
-
- Corcoran MP, Lamon-Fava S, Fielding RA. Skeletal muscle lipid deposition and insulin resistance: effect of dietary fatty acids and exercise. Am J Clin Nutr. 2007;85:662–677. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

