ATM protein kinase: the linchpin of cellular defenses to stress
- PMID: 21533982
- PMCID: PMC11115042
- DOI: 10.1007/s00018-011-0683-9
ATM protein kinase: the linchpin of cellular defenses to stress
Abstract
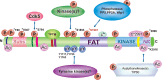
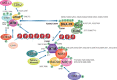
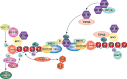
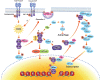
ATM is the most significant molecule involved in monitoring the genomic integrity of the cell. Any damage done to DNA relentlessly challenges the cellular machinery involved in recognition, processing and repair of these insults. ATM kinase is activated early to detect and signal lesions in DNA, arrest the cell cycle, establish DNA repair signaling and faithfully restore the damaged chromatin. ATM activation plays an important role as a barrier to tumorigenesis, metabolic syndrome and neurodegeneration. Therefore, studies of ATM-dependent DNA damage signaling pathways hold promise for treatment of a variety of debilitating diseases through the development of new therapeutics capable of modulating cellular responses to stress. In this review, we have tried to untangle the complex web of ATM signaling pathways with the purpose of pinpointing multiple roles of ATM underlying the complex phenotypes observed in AT patients.
Figures

References
-
- Shiloh Y. Ataxia-telangiectasia and the Nijmegen breakage syndrome: related disorders but genes apart. Annu Rev Genet. 1997;31:635–662. - PubMed
-
- Lavin MF. Ataxia-telangiectasia: from a rare disorder to a paradigm for cell signalling and cancer. Nat Rev Mol Cell Biol. 2008;9(10):759–769. - PubMed
-
- Savitsky K, Bar-Shira A, Gilad S, Rotman G, Ziv Y, Vanagaite L, Tagle DA, Smith S, Uziel T, Sfez S, Ashkenazi M, Pecker I, Frydman M, Harnik R, Patanjali SR, Simmons A, Clines GA, Sartiel A, Gatti RA, Chessa L, Sanal O, Lavin MF, Jaspers NG, Taylor AM, Arlett CF, Miki T, Weissman SM, Lovett M, Collins FS, Shiloh Y. A single ataxia telangiectasia gene with a product similar to PI-3 kinase. Science. 1995;268(5218):1749–1753. - PubMed
-
- Lee JH, Paull TT. Activation and regulation of ATM kinase activity in response to DNA double-strand breaks. Oncogene. 2007;26(56):7741–7748. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

