Highly diastereoselective chelation-controlled additions to α-silyloxy ketones
- PMID: 21534530
- PMCID: PMC3112462
- DOI: 10.1021/ja201629d
Highly diastereoselective chelation-controlled additions to α-silyloxy ketones
Abstract
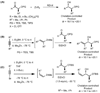
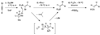
The polar Felkin-Anh, Cornforth-Evans, and Cram-chelation models predict that the addition of organometallic reagents to silyl-protected α-hydroxy ketones proceeds via a nonchelation pathway to give anti-diol addition products. This prediction has held true for the vast majority of additions reported in the literature, and few methods for chelation-controlled additions of organometallic reagents to silyl-protected α-hydroxy ketones have been introduced. Herein, we present a general and highly diastereoselective method for the addition of dialkylzincs and (E)-di-, (E)-tri-, and (Z)-disubstituted vinylzinc reagents to α-silyloxy ketones using alkyl zinc halide Lewis acids, RZnX, to give chelation-controlled products (dr ≥18:1). The compatibility of organozinc reagents with other functional groups makes this method potentially very useful in complex molecule synthesis.
Figures





Similar articles
-
Reactions of Allylmagnesium Reagents with Carbonyl Compounds and Compounds with C═N Double Bonds: Their Diastereoselectivities Generally Cannot Be Analyzed Using the Felkin-Anh and Chelation-Control Models.Chem Rev. 2020 Feb 12;120(3):1513-1619. doi: 10.1021/acs.chemrev.9b00414. Epub 2020 Jan 6. Chem Rev. 2020. PMID: 31904936 Free PMC article. Review.
-
Diastereoselective chelation-controlled additions to β-silyloxy aldehydes.Org Lett. 2012 Jul 6;14(13):3368-71. doi: 10.1021/ol301354w. Epub 2012 Jun 21. Org Lett. 2012. PMID: 22721430 Free PMC article.
-
Chelation-Controlled Additions to Chiral α- and β-Silyloxy, α-Halo, and β-Vinyl Carbonyl Compounds.Acc Chem Res. 2017 Sep 19;50(9):2389-2400. doi: 10.1021/acs.accounts.7b00319. Epub 2017 Aug 15. Acc Chem Res. 2017. PMID: 28809470
-
Overriding Felkin control: a general method for highly diastereoselective chelation-controlled additions to alpha-silyloxy aldehydes.J Am Chem Soc. 2010 Mar 31;132(12):4399-408. doi: 10.1021/ja910717p. J Am Chem Soc. 2010. PMID: 20218659 Free PMC article.
-
Catalytic asymmetric organozinc additions to carbonyl compounds.Chem Rev. 2001 Mar;101(3):757-824. doi: 10.1021/cr000411y. Chem Rev. 2001. PMID: 11712502 Review.
Cited by
-
A Highly Convergent Total Synthesis of Leustroducsin B.J Am Chem Soc. 2015 Sep 16;137(36):11594-7. doi: 10.1021/jacs.5b07438. Epub 2015 Sep 1. J Am Chem Soc. 2015. PMID: 26313159 Free PMC article.
-
Chiral ruthenium complex/Ph2P(2-furyl)-catalyzed asymmetric nucleophilic addition of aryl aldehyde hydrazones to simple ketones.Sci Adv. 2025 Mar 21;11(12):eadv0095. doi: 10.1126/sciadv.adv0095. Epub 2025 Mar 21. Sci Adv. 2025. PMID: 40117353 Free PMC article.
-
A useful methoxyvinyl cation equivalent: α-t-butyldimethylsilyl-α-methoxyacetaldehyde.Tetrahedron Lett. 2015 Jun 3;56(23):3575-3579. doi: 10.1016/j.tetlet.2015.02.122. Tetrahedron Lett. 2015. PMID: 26028786 Free PMC article.
-
Reactions of Allylmagnesium Reagents with Carbonyl Compounds and Compounds with C═N Double Bonds: Their Diastereoselectivities Generally Cannot Be Analyzed Using the Felkin-Anh and Chelation-Control Models.Chem Rev. 2020 Feb 12;120(3):1513-1619. doi: 10.1021/acs.chemrev.9b00414. Epub 2020 Jan 6. Chem Rev. 2020. PMID: 31904936 Free PMC article. Review.
-
Diastereoselective chelation-controlled additions to β-silyloxy aldehydes.Org Lett. 2012 Jul 6;14(13):3368-71. doi: 10.1021/ol301354w. Epub 2012 Jun 21. Org Lett. 2012. PMID: 22721430 Free PMC article.
References
-
- Corey EJ, Cheng X-M. The Logic of Chemical Synthesis. New York: Wiley; 1989.
-
- Nicolaou KC, Snyder SA. Classics in Total Synthesis II : More Targets, Strategies, Methods. Weinheim, Germany: Wiley-VCH; 2003.
-
- Nicolaou KC, Sorensen EJ. Classics in Total Synthesis. Weinheim, Germany: VCH; 1996.
-
- Koskinen A. Asymmetric Synthesis of Natural Products. Chichester, NY: Wiley; 1993.
-
- Guillarme S, Ple K, Banchet A, Liard A, Haudrechy A. Chem. Rev. 2006;106:2355. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

