Syntheses of α-pyrones using gold-catalyzed coupling reactions
- PMID: 21534543
- PMCID: PMC3103191
- DOI: 10.1021/ol200794w
Syntheses of α-pyrones using gold-catalyzed coupling reactions
Abstract
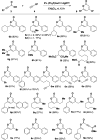
Sequential alkyne activation of terminal alkynes and propiolic acids by gold(I) catalysts yields compounds having α-pyrone skeletons. Novel cascade reactions involving propiolic acids are reported that give rise to α-pyrones with different substitution patterns.
Figures

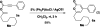

References
-
-
For the application of gold-catalyzed 6-endo-dig cyclization, see:
- Sherry B. D.; Maus L.; Laforteza B. N.; Toste F. D. J. Am. Chem. Soc. 2006, 128, 8132–8133. - PubMed
- Minnihan E. C.; Colletti S. L.; Toste F. D.; Shen H. C. J. Org. Chem. 2007, 72, 6287–6289. - PubMed
- Imase H.; Noguchi K.; Hirano M.; Tanaka K. Org. Lett. 2008, 10, 3563–3566. - PubMed
- Barabé F.; Bétournay G.; Bellavance G.; Barriault L. Org. Lett. 2009, 11, 4236–4238. - PubMed
- Menon R. S.; Findlay A. D.; Bissember A. C.; Banwell M. G. J. Org. Chem. 2009, 74, 8901–8903. - PubMed
- Jiang C.; Xu M.; Wang S.; Wang H.; Yao Z. J. J. Org. Chem. 2010, 75, 4323–4325. - PubMed
- Liu Y.; Xu W.; Wang X. Org. Lett. 2010, 12, 1448–1451. - PubMed
-
-
- Dickinson J. M. Nat. Prod. Rep. 1993, 10, 71–98. - PubMed
- van Raaij M. J.; Abrahams J. P.; Leslie A. G.; Walker J. E. Proc. Natl. Acad. Sci. U.S.A. 1996, 93, 6913–6917. - PMC - PubMed
- Steyn P. S.; van Heerden F. R. Nat. Prod. Rep. 1998, 15, 397–413. - PubMed
- Salomon C. E.; Magarvey N. A.; Sherman D. H. Nat. Prod. Rep. 2004, 21, 105–121. - PubMed
- McGlacken G. P.; Fairlamb I. J. Nat. Prod. Rep. 2005, 22, 369–385. - PubMed
- Sunazuka T.; O̅mura S. Chem. Rev. 2005, 105, 4559–4580. - PubMed
-
- Roembke P.; Schmidbaur H.; Cronje S.; Raubenheimer H. J. Mol. Catal. A 2004, 212, 35–42.
- Cui D.-M.; Meng Q.; Zheng J.-Z.; Zhang C. Chem. Commun. 2009, 1577–1579. - PubMed
- Chary B. C.; Kim S. J. Org. Chem. 2010, 75, 7928–7931. - PubMed
- Lee P. H.; Kim S.; Park A.; Chary B. C.; Kim S. Angew. Chem., Int. Ed. 2010, 49, 6806–6809. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

