Enantioselective Pd(II)-catalyzed aerobic oxidative amidation of alkenes and insights into the role of electronic asymmetry in pyridine-oxazoline ligands
- PMID: 21534607
- PMCID: PMC3103601
- DOI: 10.1021/ol200784y
Enantioselective Pd(II)-catalyzed aerobic oxidative amidation of alkenes and insights into the role of electronic asymmetry in pyridine-oxazoline ligands
Abstract
Enantioselective intramolecular oxidative amidation of alkenes has been achieved using a (pyrox)Pd(II)(TFA)(2) catalyst (pyrox = pyridine-oxazoline, TFA = trifluoroacetate) and O(2) as the sole stoichiometric oxidant. The reactions proceed at room temperature in good-to-excellent yields (58-98%) and with high enantioselectivity (ee = 92-98%). Catalyst-controlled stereoselective cyclization reactions are demonstrated for a number of chiral substrates. DFT calculations suggest that the electronic asymmetry of the pyrox ligand synergizes with steric asymmetry to control the stereochemical outcome of the key amidopalladation step.
Figures
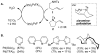


References
-
-
For reviews, see: Hegedus LS. In: Comprehensive Organic Synthesis. Semmelhack MF, editor. Vol. 4. Pergamon Press, Inc.; Elmsford: 1991. pp. 551–569. Hosokawa T, Murahashi S-I. In: Handbook of Organopalladium Chemistry for Organic Synthesis. Negishi E, de Meijere A, editors. Vol. 2. John Wiley and Sons, Inc.; New York: 2002. pp. 2169–2192. Hosokawa T. In: Handbook of Organopalladium Chemistry for Organic Synthesis. Negishi E, de Meijere A, editors. Vol. 2. John Wiley and Sons, Inc.; New York: 2002. pp. 2211–2225. Zeni G, Larock RC. Chem. Rev. 2004;104:2285–2309. Stahl SS. Angew. Chem. Int. Ed. 2004;43:3400–3420. Beccalli EM, Broggini G, Martinelli M, Sottocornola S. Chem. Rev. 2007;107:5318–5365. Minatti A, Muniz K. Chem. Soc. Rev. 2007;36:1142–1152. Kotov V, Scarborough CC, Stahl SS. Inorg. Chem. 2007;46:1910–1923. McDonald RI, Liu G, Stahl SS. Chem. Rev. 2011;111:2981–3019.
-
-
-
See the following leading references: Hosokawa T, Uno T, Inui S, Murahashi S-I. J. Am. Chem. Soc. 1981;103:2318–2323. Uozumi Y, Kato K, Hayashi T. J. Am. Chem. Soc. 1997;119:5063–5064. Uozumi Y, Kato K, Hayashi T. J. Org. Chem. 1998;63:5071–5075. Arai MA, Kuraishi M, Arai T, Sasai H. J. Am. Chem. Soc. 2001;123:2907–2908. Tsujihara T, Shinohara T, Takenaka K, Takizawa S, Onitsuka K, Hatanaka M, Sasai H. J. Org. Chem. 2009;74:9274–9279. Zhang YJ, Wang F, Zhang W. J. Org. Chem. 2007;72:9208–9213.
-
-
-
For ligand-modulated Pd-catalysts capable of using O2 as the oxidant, see: Trend RM, Ramtohul YK, Ferreira EM, Stoltz BM. Angew. Chem. Int. Ed. 2003;42:2892–2895. Trend RM, Ramtohul YK, Stoltz BM. J. Am. Chem. Soc. 2005;127:17778–17788. Yip K-T, Yang M, Law K-L, Zhu N-Y, Yang D. J. Am. Chem. Soc. 2006;128:3130–3131. He W, Yip KT, Zhu NY, Yang D. Org. Lett. 2009;11:5626–5628. Scarborough CC, Bergant A, Sazama GT, Guzei IA, Spencer LC, Stahl SS. Tetrahedron. 2009;65:5084–5092. Jiang F, Wu Z, Zhang W. Tetrahedron Lett. 2010;51:5124–5126.
-
-
-
For important advances in related enantioselective metal-catalyzed C–N bond forming reactions, see the following review and selected primary references: Chemler SR. Org. Biomol. Chem. 2009;7:3009–3019. Overman LE, Remarchuk TP. J. Am. Chem. Soc. 2002;124:12–13. Mai DN, Wolfe JP. J. Am. Chem. Soc. 2010;132:12157–12159. Du H, Zhao B, Shi Y. J. Am. Chem. Soc. 2008;130:8590–8591.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

