The incidence, predictors and management of anaemia and its association with virological response in HCV / HIV coinfected persons treated with long-term pegylated interferon alfa 2a and ribavirin
- PMID: 21535051
- PMCID: PMC3184244
- DOI: 10.1111/j.1365-2036.2011.04648.x
The incidence, predictors and management of anaemia and its association with virological response in HCV / HIV coinfected persons treated with long-term pegylated interferon alfa 2a and ribavirin
Abstract
Background: The association of anaemia with outcomes in the HCV/HIV coinfected persons undergoing HCV treatment remains unclear.
Aims: To study the incidence, predictors and management of anaemia, and its association with outcomes among persons treated with pegylated interferon and weight-based ribavirin.
Methods: Retrospective analysis of a prospective controlled treatment trial of HCV/HIV coinfection.
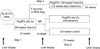
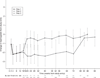
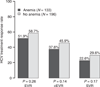
Results: Among 329 subjects enrolled, 40% developed anaemia during the first 12-18 weeks of treatment (median haemoglobin decrease at week 4: 2.2 g/dL). Among 169 subjects who achieved early virological response and received therapy for 72 weeks, 55% eventually developed anaemia. However, median haemoglobin levels stayed stable after 12-18 weeks of initial therapy. Among these 169 subjects, 45% were prescribed an erythropoiesis stimulating agent (ESA), with 17% receiving it prior to a drop in haemoglobin meeting protocol definition of anaemia. Only 27% completed the study without any ribavirin dose modification. Age >40 years, lower BMI, zidovudine use and lower entry haemoglobin were significant predictors of anaemia in the multi-covariate model. Among all 329, sustained virological response (SVR) rate was similar in those with or without anaemia (23% vs. 30%; P=0.17) with no evidence of association between anaemia or ESA use and treatment response.
Conclusions: Anaemia is common in HCV/HIV coinfected persons undergoing HCV treatment, and only a minority of them are able to maintain ribavirin dose. Persons with age >40 years, lower baseline haemoglobin and lower baseline BMI should be monitored carefully. Prescription of erythropoiesis stimulating agent is common, but anaemia or erythropoiesis stimulating agent use is not associated with SVR.
© 2011 Blackwell Publishing Ltd.
Figures


References
-
- Sherman KE, Rouster SD, Chung RT, Rajicic N. Hepatitis C virus prevalence among patients infected with human immunodeficiency virus: a cross-sectional analysis of the US adult AIDS Clinical Trials Group. Clin Infect Dis. 2002;34:831–837. - PubMed
-
- Justice AC, Lasky E, McGinnis KA, et al. Medical disease and alcohol use among veterans with human immunodeficiency infection: a comparison of disease measurement strategies. Med Care. 2006;44(8) Suppl. 2:S52–S60. - PubMed
-
- Torriani FJ, Rodriguez-Torres M, Rockstroh JK, et al. Peginterferon Alfa-2a plus ribavirin for chronic hepatitis C virus infection in HIV-infected patients. N Engl J Med. 2004;351:438–450. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
