Functional cooperation between the proteins Nck and ADAP is fundamental for actin reorganization
- PMID: 21536650
- PMCID: PMC3133383
- DOI: 10.1128/MCB.01358-10
Functional cooperation between the proteins Nck and ADAP is fundamental for actin reorganization
Abstract
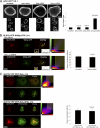
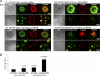
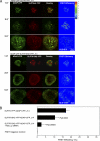
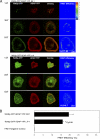
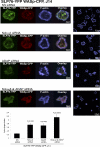
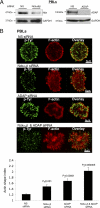
T cell antigen receptor (TCR) activation triggers profound changes in the actin cytoskeleton. In addition to controlling cellular shape and polarity, this process regulates vital T cell responses, such as T cell adhesion, motility, and proliferation. These depend on the recruitment of the signaling proteins Nck and Wiskott-Aldrich syndrome protein (WASp) to the site of TCR activation and on the functional properties of the adapter proteins linker for activation of T cells (LAT) and SH2-domain-containing leukocyte protein of 76 kDa (SLP76). We now demonstrate that Nck is necessary but insufficient for the recruitment of WASp. We show that two pathways lead to SLP76-dependent actin rearrangement. One requires the SLP76 acidic domain, crucial to association with the Nck SH2 domain, and another requires the SLP76 SH2 domain, essential for interaction with the adhesion- and degranulation-promoting adapter protein ADAP. Functional cooperation between Nck and ADAP mediates SLP76-WASp interactions and actin rearrangement. We also reveal the molecular mechanism linking ADAP to actin reorganization.
Figures

References
-
- Badour K., et al. 2003. The Wiskott-Aldrich syndrome protein acts downstream of CD2 and the CD2AP and PSTPIP1 adaptors to promote formation of the immunological synapse. Immunity 18:141–154 - PubMed
-
- Badour K., Zhang J., Siminovitch K. A. 2004. Involvement of the Wiskott-Aldrich syndrome protein and other actin regulatory adaptors in T cell activation. Semin. Immunol. 16:395–407 - PubMed
-
- Barda-Saad M., et al. 2005. Dynamic molecular interactions linking the T cell antigen receptor to the actin cytoskeleton. Nat. Immunol. 6:80–89 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
