Oncogenic tyrosine kinases target Dok-1 for ubiquitin-mediated proteasomal degradation to promote cell transformation
- PMID: 21536658
- PMCID: PMC3133381
- DOI: 10.1128/MCB.05045-11
Oncogenic tyrosine kinases target Dok-1 for ubiquitin-mediated proteasomal degradation to promote cell transformation
Abstract
Cellular transformation induced by oncogenic tyrosine kinases is a multistep process involving activation of growth-promoting signaling pathways and inactivation of suppressor molecules. Dok-1 is an adaptor protein that acts as a negative regulator of tyrosine kinase-initiated signaling and opposes oncogenic tyrosine kinase-mediated cell transformation. Findings that its loss facilitates transformation induced by oncogenic tyrosine kinases suggest that Dok-1 inactivation could constitute an intermediate step in oncogenesis driven by these oncoproteins. However, whether Dok-1 is subject to regulation by oncogenic tyrosine kinases remained unknown. In this study, we show that oncogenic tyrosine kinases, including p210(bcr-abl) and oncogenic forms of Src, downregulate Dok-1 by targeting it for degradation through the ubiquitin-proteasome pathway. This process is dependent on the tyrosine kinase activity of the oncoproteins and is mediated primarily by lysine-dependent polyubiquitination of Dok-1. Importantly, restoration of Dok-1 levels strongly suppresses transformation of cells expressing oncogenic tyrosine kinases, and this suppression is more pronounced in the context of a Dok-1 mutant that is largely refractory to oncogenic tyrosine kinase-induced degradation. Our findings suggest that proteasome-mediated downregulation of Dok-1 is a key mechanism by which oncogenic tyrosine kinases overcome the inhibitory effect of Dok-1 on cellular transformation and tumor progression.
Figures


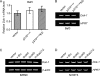
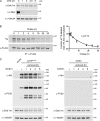
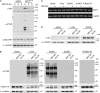

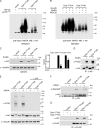
References
-
- Abram C. L., Courtneidge S. A. 2000. Src family tyrosine kinases and growth factor signaling. Exp. Cell Res. 254:1–13 - PubMed
-
- Barnes D. J., Schultheis B., Adedeji S., Melo J. V. 2005. Dose-dependent effects of Bcr-Abl in cell line models of different stages of chronic myeloid leukemia. Oncogene 24:6432–6440 - PubMed
-
- Berman E., et al. 2000. Characterization of two novel sublines established from a human megakaryoblastic leukemia cell line transfected with p210(BCR-ABL). Leuk. Res. 24:289–297 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
