Survivin monomer plays an essential role in apoptosis regulation
- PMID: 21536684
- PMCID: PMC3123095
- DOI: 10.1074/jbc.M111.237586
Survivin monomer plays an essential role in apoptosis regulation
Abstract
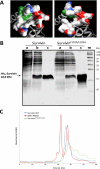
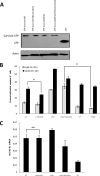
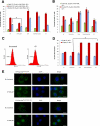
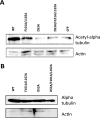
Survivin was initially described as an inhibitor of apoptosis and attracted growing attention as one of the most tumor-specific genes in the human genome and a promising target for cancer therapy. Lately, it has been shown that survivin is a multifunctional protein that takes part in several crucial cell processes. At first, it was supposed that survivin functions only as a homodimer, but now data indicate that many processes require monomeric survivin. Moreover, recent studies reveal a special mechanism regulating the balance between monomeric and dimeric forms of the protein. In this paper we studied the mutant form of survivin that was unable to dimerize and investigated its role in apoptosis. We showed that survivin monomer interacts with Smac/DIABLO and X-linked inhibitor of apoptosis protein (XIAP) both in vitro and in vivo. Due to this feature, it protects cells from caspase-dependent apoptosis even more efficiently than the wild-type survivin. We also identified that mutant monomeric survivin prevents apoptosis-inducing factor release from the mitochondrial intermembrane space, protecting human fibrosarcoma HT1080 cells from caspase-independent apoptosis. On the other hand, our results indicate that only wild-type survivin, but not the monomer mutant form, enhances tubulin stability in cells. These findings suggest that survivin partly performs its functions as a monomer and partly as a dimer. The mechanism of dimer-monomer balance regulation may also work as a "switcher" between survivin functions and thereby explain remarkable functional diversities of this protein.
Figures

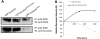


References
-
- Ambrosini G., Adida C., Altieri D. C. (1997) Nat. Med. 3, 917–921 - PubMed
-
- Velculescu V. E., Madden S. L., Zhang L., Lash A. E., Yu J., Rago C., Lal A., Wang C. J., Beaudry G. A., Ciriello K. M., Cook B. P., Dufault M. R., Ferguson A. T., Gao Y., He T. C., Hermeking H., Hiraldo S. K., Hwang P. M., Lopez M. A., Luderer H. F., Mathews B., Petroziello J. M., Polyak K., Zawel L., Kinzler K. W. (1999) Nat. Genet. 23, 387–388 - PubMed
-
- Altieri D. C. (2008) Nat. Rev. Cancer 8, 61–70 - PubMed
-
- Lens S. M., Medema R. H. (2003) Cell Cycle 2, 507–510 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

