The stress of protein misfolding: from single cells to multicellular organisms
- PMID: 21536706
- PMCID: PMC3098679
- DOI: 10.1101/cshperspect.a009704
The stress of protein misfolding: from single cells to multicellular organisms
Abstract
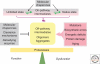
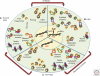
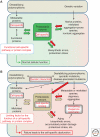
Organisms survive changes in the environment by altering their rates of metabolism, growth, and reproduction. At the same time, the system must ensure the stability and functionality of its macromolecules. Fluctuations in the environment are sensed by highly conserved stress responses and homeostatic mechanisms, and of these, the heat shock response (HSR) represents an essential response to acute and chronic proteotoxic damage. However, unlike the strategies employed to maintain the integrity of the genome, protection of the proteome must be tailored to accommodate the normal flux of nonnative proteins and the differences in protein composition between cells, and among individuals. Moreover, adult cells are likely to have significant differences in the rates of synthesis and clearance that are influenced by intrinsic errors in protein expression, genetic polymorphisms, and fluctuations in physiological and environmental conditions. Here, we will address how protein homeostasis (proteostasis) is achieved at the level of the cell and organism, and how the threshold of the stress response is set to detect and combat protein misfolding. For metazoans, the requirement for coordinated function and growth imposes additional constraints on the detection, signaling, and response to misfolding, and requires that the HSR is integrated into various aspects of organismal physiology, such as lifespan. This is achieved by hierarchical regulation of heat shock factor 1 (HSF1) by the metabolic state of the cell and centralized neuronal control that could allow optimal resource allocation between cells and tissues. We will examine how protein folding quality control mechanisms in individual cells may be integrated into a multicellular level of control, and further, even custom-designed to support individual variability and impose additional constraints on evolutionary adaptation.
Figures

References
-
- Abravaya K, Phillips B, Morimoto RI 1991. Attenuation of the heat shock response in HeLa cells is mediated by the release of bound heat shock transcription factor and is modulated by changes in growth and in heat shock temperatures. Genes Dev 5: 2117–2127 - PubMed
-
- Abravaya K, Myers MP, Murphy SP, Morimoto RI 1992. The human heat shock protein hsp70 interacts with HSF, the transcription factor that regulates heat shock gene expression. Genes Dev 6: 1153–1164 - PubMed
-
- Alcedo J, Kenyon C 2004. Regulation of C. elegans longevity by specific gustatory and olfactory neurons. Neuron 41: 45–55 - PubMed
-
- Allali-Hassani A, Wasney GA, Chau I, Hong BS, Senisterra G, Loppnau P, Shi Z, Moult J, Edwards AM, Arrowsmith CH, et al. 2009. A survey of proteins encoded by non-synonymous single nucleotide polymorphisms reveals a significant fraction with altered stability and activity. Biochem J 424: 15–26 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
