Gain-of-function mutations in interleukin-7 receptor-α (IL7R) in childhood acute lymphoblastic leukemias
- PMID: 21536738
- PMCID: PMC3092356
- DOI: 10.1084/jem.20110580
Gain-of-function mutations in interleukin-7 receptor-α (IL7R) in childhood acute lymphoblastic leukemias
Erratum in
- J Exp Med. 2011 Jun 6;208(6):1333
- J Exp Med. 2011 May 9;208(5):preceding 901
Abstract
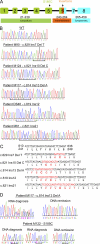
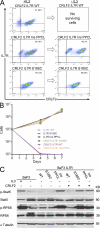
Interleukin-7 receptor α (IL7R) is required for normal lymphoid development. Loss-of-function mutations in this gene cause autosomal recessive severe combined immune deficiency. Here, we describe somatic gain-of-function mutations in IL7R in pediatric B and T acute lymphoblastic leukemias. The mutations cause either a serine-to-cysteine substitution at amino acid 185 in the extracellular domain (4 patients) or in-frame insertions and deletions in the transmembrane domain (35 patients). In B cell precursor leukemias, the mutations were associated with the aberrant expression of cytokine receptor-like factor 2 (CRLF2), and the mutant IL-7R proteins formed a functional receptor with CRLF2 for thymic stromal lymphopoietin (TSLP). Biochemical and functional assays reveal that these IL7R mutations are activating mutations conferring cytokine-independent growth of progenitor lymphoid cells. A cysteine, included in all but three of the mutated IL-7R alleles, is essential for the constitutive activation of the receptor. This is the first demonstration of gain-of-function mutations of IL7R. Our current and recent observations of mutations in IL7R and CRLF2, respectively suggest that the addition of cysteine to the juxtamembranous domains is a general mechanism for mutational activation of type I cytokine receptors in leukemia.
Figures
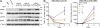

References
-
- Cario G., Zimmermann M., Romey R., Gesk S., Vater I., Harbott J., Schrauder A., Moericke A., Izraeli S., Akasaka T., et al. 2010. Presence of the P2RY8-CRLF2 rearrangement is associated with a poor prognosis in non-high-risk precursor B-cell acute lymphoblastic leukemia in children treated according to the ALL-BFM 2000 protocol. Blood. 115:5393–5397 10.1182/blood-2009-11-256131 - DOI - PubMed
-
- Clappier E., Collette S., Grardel N., Girard S., Suarez L., Brunie G., Kaltenbach S., Yakouben K., Mazingue F., Robert A., et al. ; EORTC-CLG 2010. NOTCH1 and FBXW7 mutations have a favorable impact on early response to treatment, but not on outcome, in children with T-cell acute lymphoblastic leukemia (T-ALL) treated on EORTC trials 58881 and 58951. Leukemia. 24:2023–2031 10.1038/leu.2010.205 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

