Trypanosoma cruzi subverts the sphingomyelinase-mediated plasma membrane repair pathway for cell invasion
- PMID: 21536739
- PMCID: PMC3092353
- DOI: 10.1084/jem.20102518
Trypanosoma cruzi subverts the sphingomyelinase-mediated plasma membrane repair pathway for cell invasion
Abstract
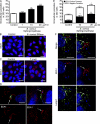
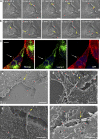
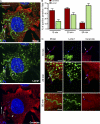
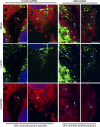
Upon host cell contact, the protozoan parasite Trypanosoma cruzi triggers cytosolic Ca(2+) transients that induce exocytosis of lysosomes, a process required for cell invasion. However, the exact mechanism by which lysosomal exocytosis mediates T. cruzi internalization remains unclear. We show that host cell entry by T. cruzi mimics a process of plasma membrane injury and repair that involves Ca(2+)-dependent exocytosis of lysosomes, delivery of acid sphingomyelinase (ASM) to the outer leaflet of the plasma membrane, and a rapid form of endocytosis that internalizes membrane lesions. Host cells incubated with T. cruzi trypomastigotes are transiently wounded, show increased levels of endocytosis, and become more susceptible to infection when injured with pore-forming toxins. Inhibition or depletion of lysosomal ASM, which blocks plasma membrane repair, markedly reduces the susceptibility of host cells to T. cruzi invasion. Notably, extracellular addition of sphingomyelinase stimulates host cell endocytosis, enhances T. cruzi invasion, and restores normal invasion levels in ASM-depleted cells. Ceramide, the product of sphingomyelin hydrolysis, is detected in newly formed parasitophorous vacuoles containing trypomastigotes but not in the few parasite-containing vacuoles formed in ASM-depleted cells. Thus, T. cruzi subverts the ASM-dependent ceramide-enriched endosomes that function in plasma membrane repair to infect host cells.
Figures

Comment in
-
Parasitology: Adding insult to injury.Nat Rev Microbiol. 2011 May 31;9(7):484. doi: 10.1038/nrmicro2597. Nat Rev Microbiol. 2011. PMID: 21625249 No abstract available.
Similar articles
-
Membrane cholesterol regulates lysosome-plasma membrane fusion events and modulates Trypanosoma cruzi invasion of host cells.PLoS Negl Trop Dis. 2012;6(3):e1583. doi: 10.1371/journal.pntd.0001583. Epub 2012 Mar 27. PLoS Negl Trop Dis. 2012. PMID: 22479662 Free PMC article.
-
Solving the secretory acid sphingomyelinase puzzle: Insights from lysosome-mediated parasite invasion and plasma membrane repair.Cell Microbiol. 2019 Nov;21(11):e13065. doi: 10.1111/cmi.13065. Epub 2019 Jun 10. Cell Microbiol. 2019. PMID: 31155842 Free PMC article. Review.
-
The exocyst is required for trypanosome invasion and the repair of mechanical plasma membrane wounds.J Cell Sci. 2015 Jan 1;128(1):27-32. doi: 10.1242/jcs.150573. Epub 2014 Nov 6. J Cell Sci. 2015. PMID: 25380822 Free PMC article.
-
Exocytosis of acid sphingomyelinase by wounded cells promotes endocytosis and plasma membrane repair.J Cell Biol. 2010 Jun 14;189(6):1027-38. doi: 10.1083/jcb.201003053. Epub 2010 Jun 7. J Cell Biol. 2010. PMID: 20530211 Free PMC article.
-
Don't bother to knock--the cell invasion strategy of Trypanosoma cruzi.Trends Parasitol. 2002 Oct;18(10):427-8. doi: 10.1016/s1471-4922(02)02368-1. Trends Parasitol. 2002. PMID: 12377585 Review.
Cited by
-
Altered Clathrin-Independent Endocytosis in Type A Niemann-Pick Disease Cells and Rescue by ICAM-1-Targeted Enzyme Delivery.Mol Pharm. 2015 May 4;12(5):1366-76. doi: 10.1021/mp5005959. Epub 2015 Apr 23. Mol Pharm. 2015. PMID: 25849869 Free PMC article.
-
Plasma membrane damage repair is mediated by an acid sphingomyelinase in Entamoeba histolytica.PLoS Pathog. 2019 Aug 28;15(8):e1008016. doi: 10.1371/journal.ppat.1008016. eCollection 2019 Aug. PLoS Pathog. 2019. PMID: 31461501 Free PMC article.
-
Membrane cholesterol regulates lysosome-plasma membrane fusion events and modulates Trypanosoma cruzi invasion of host cells.PLoS Negl Trop Dis. 2012;6(3):e1583. doi: 10.1371/journal.pntd.0001583. Epub 2012 Mar 27. PLoS Negl Trop Dis. 2012. PMID: 22479662 Free PMC article.
-
The Exocyst Complex in Health and Disease.Front Cell Dev Biol. 2016 Apr 12;4:24. doi: 10.3389/fcell.2016.00024. eCollection 2016. Front Cell Dev Biol. 2016. PMID: 27148529 Free PMC article. Review.
-
Cell signaling during Trypanosoma cruzi invasion.Front Immunol. 2012 Nov 28;3:361. doi: 10.3389/fimmu.2012.00361. eCollection 2012. Front Immunol. 2012. PMID: 23230440 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

