sGC{alpha}1 mediates the negative inotropic effects of NO in cardiac myocytes independent of changes in calcium handling
- PMID: 21536853
- PMCID: PMC3129918
- DOI: 10.1152/ajpheart.01273.2010
sGC{alpha}1 mediates the negative inotropic effects of NO in cardiac myocytes independent of changes in calcium handling
Abstract
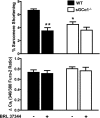
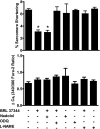
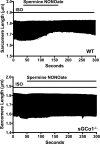
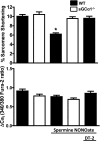
In the heart, nitric oxide (NO) modulates contractile function; however, the mechanisms responsible for this effect are incompletely understood. NO can elicit effects via a variety of mechanisms including S-nitrosylation and stimulation of cGMP synthesis by soluble guanylate cyclase (sGC). sGC is a heterodimer comprised of a β(1)- and an α(1)- or α(2)-subunit. sGCα(1)β(1) is the predominant isoform in the heart. To characterize the role of sGC in the regulation of cardiac contractile function by NO, we compared left ventricular cardiac myocytes (CM) isolated from adult mice deficient in the sGC α(1)-subunit (sGCα(1)(-/-)) and from wild-type (WT) mice. Sarcomere shortening under basal conditions was less in sGCα(1)(-/-) CM than in WT CM. To activate endogenous NO synthesis from NO synthase 3, CM were incubated with the β(3)-adrenergic receptor (β(3)-AR) agonist BRL 37344. BRL 37344 decreased cardiac contractility in WT CM but not in sGCα(1)(-/-) myocytes. Administration of spermine NONOate, an NO donor compound, did not affect sarcomeric shortening in CM of either genotype; however, in the presence of isoproterenol, addition of spermine NONOate reduced sarcomere shortening in WT but not in sGCα(1)(-/-) CM. Neither BRL 37344 nor spermine NONOate altered calcium handling in CM of either genotype. These findings suggest that sGCα(1) exerts a positive inotropic effect under basal conditions, as well as mediates the negative inotropic effect of β(3)-AR signaling. Additionally, our work demonstrates that sGCα(1)β(1) is required for NO to depress β(1)/β(2)-AR-stimulated cardiac contractility and that this modulation is independent of changes in calcium handling.
Figures

References
-
- Barouch LA, Harrison RW, Skaf MW, Rosas GO, Cappola TP, Kobeissi ZA, Hobai IA, Lemmon CA, Burnett AL, O'Rourke B, Rodriguez ER, Huang PL, Lima JA, Berkowitz DE, Hare JM. Nitric oxide regulates the heart by spatial confinement of nitric oxide synthase isoforms. Nature 416: 337–339, 2002 - PubMed
-
- Boivin B, Lavoie C, Vaniotis G, Baragli A, Villeneuve LR, Ethier N, Trieu P, Allen BG, Hebert TE. Functional beta-adrenergic receptor signaling on nuclear membranes in adult rat and mouse ventricular cardiomyocytes. Cardiovasc Res 71: 69–78, 2006 - PubMed
-
- Borlaug BA, Melenovsky V, Marhin T, Fitzgerald P, Kass DA. Sildenafil inhibits beta-adrenergic-stimulated cardiac contractility in humans. Circulation 112: 2642–2649, 2005 - PubMed
-
- Budworth J, Meillerais S, Charles I, Powell K. Tissue distribution of the human soluble guanylate cyclases. Biochem Biophys Res Commun 263: 696–701, 1999 - PubMed
-
- Buys ES, Sips P, Vermeersch P, Raher MJ, Rogge E, Ichinose F, Dewerchin M, Bloch KD, Janssens S, Brouckaert P. Gender-specific hypertension and responsiveness to nitric oxide in sGCalpha1 knockout mice. Cardiovasc Res 79: 179–186, 2008 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

