Regulation of angiogenesis and choroidal neovascularization by members of microRNA-23~27~24 clusters
- PMID: 21536891
- PMCID: PMC3100947
- DOI: 10.1073/pnas.1105254108
Regulation of angiogenesis and choroidal neovascularization by members of microRNA-23~27~24 clusters
Abstract
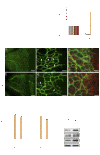
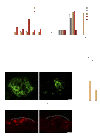
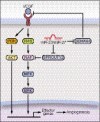
MicroRNAs (miRNAs) modulate complex physiological and pathological processes by repressing expression of multiple components of cellular regulatory networks. Here we demonstrate that miRNAs encoded by the miR-23∼27∼24 gene clusters are enriched in endothelial cells and highly vascularized tissues. Inhibition of miR-23 and miR-27 function by locked nucleic acid-modified anti-miRNAs represses angiogenesis in vitro and postnatal retinal vascular development in vivo. Moreover, miR-23 and miR-27 are required for pathological angiogenesis in a laser-induced choroidal neovascularization mouse model. MiR-23 and miR-27 enhance angiogenesis by promoting angiogenic signaling through targeting Sprouty2 and Sema6A proteins, which exert antiangiogenic activity. Manipulating miR-23/27 levels may have important therapeutic implications in neovascular age-related macular degeneration and other vascular disorders.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Distler JH, et al. Angiogenic and angiostatic factors in the molecular control of angiogenesis. Q J Nucl Med. 2003;47:149–161. - PubMed
-
- Jager RD, Mieler WF, Miller JW. Age-related macular degeneration. N Engl J Med. 2008;358:2606–2617. - PubMed
-
- Grisanti S, Tatar O. The role of vascular endothelial growth factor and other endogenous interplayers in age-related macular degeneration. Prog Retin Eye Res. 2008;27:372–390. - PubMed
-
- Brown DM, et al. ANCHOR Study Group. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1432–1444. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

