Fluctuating and sensory-induced vasodynamics in rodent cortex extend arteriole capacity
- PMID: 21536897
- PMCID: PMC3100929
- DOI: 10.1073/pnas.1100428108
Fluctuating and sensory-induced vasodynamics in rodent cortex extend arteriole capacity
Abstract
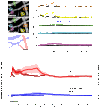
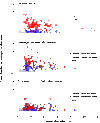
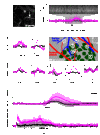
Neural activity in the brain is followed by localized changes in blood flow and volume. We address the relative change in volume for arteriole vs. venous blood within primary vibrissa cortex of awake, head-fixed mice. Two-photon laser-scanning microscopy was used to measure spontaneous and sensory evoked changes in flow and volume at the level of single vessels. We find that arterioles exhibit slow (<1 Hz) spontaneous increases in their diameter, as well as pronounced dilation in response to both punctate and prolonged stimulation of the contralateral vibrissae. In contrast, venules dilate only in response to prolonged stimulation. We conclude that stimulation that occurs on the time scale of natural stimuli leads to a net increase in the reservoir of arteriole blood. Thus, a "bagpipe" model that highlights arteriole dilation should augment the current "balloon" model of venous distension in the interpretation of fMRI images.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Ogawa S, Lee TM, Nayak AS, Glynn P. Oxygenation-sensitive contrast in magnetic resonance image of rodent brain at high magnetic fields. Magn Reson Med. 1990;14:68–78. - PubMed
-
- Grinvald A, Lieke EE, Frostig RD, Gilbert CD, Wiesel TN. Functional architecture of cortex revealed by optical imaging of intrinsic signals. Nature. 1986;324:361–364. - PubMed
-
- Logothetis NK, Wandell BA. Interpreting the BOLD signal. Annu Rev Physiol. 2004;66:735–769. - PubMed
-
- Buxton RB, Wong EC, Frank LR. Dynamics of blood flow and oxygenation changes during brain activation: the balloon model. Magn Reson Med. 1998;39:855–864. - PubMed
-
- Mandeville JB, et al. Evidence of a cerebrovascular postarteriole windkessel with delayed compliance. J Cereb Blood Flow Metab. 1999;19:679–689. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

